Abstract
Research Article
Budesonide – Oral Galenic Formulations for Crohn Disease
Luisetto M, Mashori GR, Cabianca L and Latyshev OYU
Published: 11 October, 2024 | Volume 8 - Issue 1 | Pages: 028-033
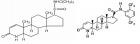
The aim of this work is to verify the pharmaceutical form in the galenic field of oral Budesonide compounded used in Crohn’s disease: capsules delay release or oral suspension. In particular ways the kinds of excipients or bases-vehicle used in the galenic pharmacy practice. The therapeutic need for Crohn’s disease requires a release of the API in delayed-release DR. The Budesonide molecule shows low systemic impacts due to its hepatic metabolism vs. a topical effect useful in this pathology. In this work, the oral pharmaceutical forms are analyzed: modified-release capsules and oral suspension with specific advantages for each one. Some formulations provided by various pharmacies are reported in this work as well as new technology like the 3D-PRINTING systems for colonic targeting tablets.
Read Full Article HTML DOI: 10.29328/journal.acgh.1001048 Cite this Article Read Full Article PDF
Keywords:
Budesonide; Crohn's disease; Pediatric; Delay release; Capsules acid resistance filled with HPMC; Metolose; Methocel; Oral suspension; Ready for use vehicle; 3D printing
References
- Kelsen J, Baldassano R N. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008;14(suppl_2):S9-11. Available from: https://doi.org/10.1002/ibd.20560
- Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017;92(7):1088-1103. Available from: https://doi.org/10.1016/j.mayocp.2017.04.010
- Brandi Jones M-E, Rekhi S. What’s the difference between Crohn’s disease and ulcerative colitis? Health. 2024 Feb 7. Available from: https://www.health.com/crohns-disease-vs-ulcerative-colitis-8405457
- Crohn’s Disease. Johns Hopkins Medicine. 2022 Apr 19. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/crohns-disease
- von Allmen D. Pediatric Crohn's disease. Pediatr Congenit Colorectal Dis Adult Patient. 2018;31(2):80-88. Available from: https://doi.org/10.1055/s-0037-1609022
- Kumar A, Cole A, Segal J, Smith P, Limdi JK. A review of the therapeutic management of Crohn’s disease. Ther Adv Gastroenterol. 2022;15:17562848221078456. Available from: https://doi.org/10.1177/17562848221078456
- Greenberg G R, Feagan B G, Martin F, Sutherland L R, Thomson A, Williams C N, et al. Oral budesonide for active Crohn's disease. N Engl J Med. 1994;331(13):836-841. Available from: https://doi.org/10.1056/nejm199409293311303
- Budesonide 2 mg/10 ml Oral Suspension | Formulas | Bayview Pharmacy. Available from: https://www.bayviewrx.com/formulas/Budesonide-2-mg-10-ml-Oral-Suspension-Asthma-Allergic-Rhinitis-Crohn-s-Disease-Ulcerative-Colitis-Eosinophilic-Esophagitis
- Budesonide (oral route). Mayo Clinic. 2024 Oct 1. Available from: https://www.mayoclinic.org/drugs-supplements/budesonide-oral-route/description/drg-20073233
- Budesonide Te Arai. Budesonide Te Arai Data Sheet v 3.0 July 2023. Available from: https://www.medsafe.govt.nz/profs/datasheet/b/BudesonideTeAraicap.pdf
- Hennig M, Hennig M. Colon-targeted delivery systems of budesonide as second-line therapy in inflammatory bowel disease. Pharma Excipients. 2024 Feb 25. Available from: https://www.pharmaexcipients.com/news/budesonide-second-line/
- O'Donnell S, O'Morain CA. Therapeutic benefits of budesonide in gastroenterology. Ther Adv Chronic Dis. 2010;1(4):177-186. Available from: https://doi.org/10.1177/2040622310379293
- Budesonide 10 mg Slow Release Acid Resistant Capsules | Formulas | Bayview Pharmacy. Available from: https://www.bayviewrx.com/formulas/Budesonide-10-mg-Slow-Release-Acid-Resistant-Capsules-Asthma-Crohn-s-Disease-Ulcerative-Colitis-Allergic-Rhinitis-Eosinophilic-Esophagitis
- Rowe RC, Sheskey PJ, Owen SC. Handbook of Pharmaceutical Excipients. 5th ed. Pharmaceutical Press; 2006;918. https://books.google.co.in/books/about/Handbook_of_Pharmaceutical_Excipients.html?id=BGJqAAAAMAAJ
- Yokoyama T, Ohta A, Motoya S, Takazoe M, Yajima T, Date M, et al. Efficacy and safety of oral budesonide in patients with active Crohn's disease in Japan: a multicenter, double-blind, randomized, parallel-group phase 3 study. Inflamm Intest Dis. 2018;2(3):154-162. Available from: https://doi.org/10.1159/000484047
- Chopra A, Pardi DS, Loftus EV, Tremaine WJ, Egan LJ, Faubion WA, et al. Budesonide in the treatment of inflammatory bowel disease: the first year of experience in clinical practice. Inflamm Bowel Dis. 2006;12(1):29-32. Available from: https://doi.org/10.1097/01.mib.0000192323.82426.83
- Ou YH, Goh WJ, Lim SH. Form & formulation approaches for COntRollable Release in 3D printed Colonic Targeting (CORR3CT) budesonide tablet. Int J Pharm. 2023;635:122680. Available from: https://doi.org/10.1016/j.ijpharm.2023.122680
- Cortesi R, Ravani L, Menegatti E, Esposito E, Ronconi F. Eudragit® microparticles for the release of budesonide: a comparative study. Indian J Pharm Sci. 2012;74(5):415-421. Available from: https://doi.org/10.4103/0250-474x.108416
- Iborra M, Álvarez-Sotomayor D, Nos P. Long-term safety and efficacy of budesonide in the treatment of ulcerative colitis. Clin Exp Gastroenterol. 2014;39-46. Available from: https://doi.org/10.2147/ceg.s34715
- Dressman J. Comparative dissolution of Budesonide from four commercially available products for oral administration: implications for interchangeability. Dissol Technol. 2023;30:224-229. Available from: https://dissolutiontech.com/issues/202311/DT202311_A02.pdf
- MMXTM technology and its applications in gastrointestinal diseases - Tasnim Pharmaceutical Company. Tasnim Pharmaceutical Company. 2023 Jul 19. Available from: https://tasnimpharma.com/mmx-technology/
- Moghrabi FS, Fadda HM. Drug physicochemical properties and capsule fill determine extent of premature gastric release from enteric capsules. Pharmaceutics. 2022;14(11):2505. Available from: https://doi.org/10.3390/pharmaceutics14112505
- Shin-Etsu technical information - SE Tylose. Extended Release of Vitamin C Matrix Tablets with TYLOPUR Xtend Nutra®. Available from: https://www.setylose.com/en/knowledge-base/healthcare/technical-information#tylopur-xtend-nutra-10231
Figures:
Similar Articles
-
Treatment of perianal fistulae in crohn's disease with mesenchymal stem cellsMohamed Hassin Mohamed Chairi*,Francisco José Huertas Peña,Marta Santidrián Zurbano,Tomás Torres Alcalá,Jesús María Villar del Moral. Treatment of perianal fistulae in crohn's disease with mesenchymal stem cells. . 2022 doi: 10.29328/journal.acgh.1001033; 6: 006-020
-
Budesonide – Oral Galenic Formulations for Crohn DiseaseLuisetto M, Mashori GR, Cabianca L, Latyshev OYU. Budesonide – Oral Galenic Formulations for Crohn Disease. . 2024 doi: 10.29328/journal.acgh.1001048; 8: 028-033
Recently Viewed
-
Metastatic Brain Melanoma: A Rare Case with Review of LiteratureNeha Singh,Gaurav Raj,Akshay Kumar,Deepak Kumar Singh,Shivansh Dixit,Kaustubh Gupta*. Metastatic Brain Melanoma: A Rare Case with Review of Literature. J Radiol Oncol. 2025: doi: ; 9: 050-053
-
Depression as a civilization-deformed adaptation and defence mechanismBohdan Wasilewski*,Olha Yourtsenyuk,Eugene Egan. Depression as a civilization-deformed adaptation and defence mechanism. Insights Depress Anxiety. 2020: doi: 10.29328/journal.ida.1001013; 4: 008-011
-
Drinking-water Quality Assessment in Selective Schools from the Mount LebanonWalaa Diab, Mona Farhat, Marwa Rammal, Chaden Moussa Haidar*, Ali Yaacoub, Alaa Hamzeh. Drinking-water Quality Assessment in Selective Schools from the Mount Lebanon. Ann Civil Environ Eng. 2024: doi: 10.29328/journal.acee.1001061; 8: 018-024
-
Rapid Microbial Growth in Reusable Drinking Water BottlesQishan Liu*,Hongjun Liu. Rapid Microbial Growth in Reusable Drinking Water Bottles. Ann Civil Environ Eng. 2017: doi: 10.29328/journal.acee.1001007; 1: 055-062
-
Beneficial effects of a ketogenic diet in a woman with Charcot-Marie-Tooth diseaseElvira Rostanzo,Anna Maria Aloisi*. Beneficial effects of a ketogenic diet in a woman with Charcot-Marie-Tooth disease. Arch Food Nutr Sci. 2022: doi: 10.29328/journal.afns.1001040; 6: 068-072
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."