Case Report
An uncommon cause of isolated ascites: Pseudomyxoma peritonei

Louly Hady1*, I Nassar2, K Znati3 and N Kabbaj1
1EFD-HGE Department, Ibn Sina Hospital, Rabat, Morocco
2Central Radiology Department, Ibn Sina Hospital, Rabat, Morocco
3Central Anatomical Pathology Department, Ibn Sina Hospital, Rabat, Morocco
*Address for Correspondence: Louly Hady, EFD-HGE Department, Ibn Sina Hospital, Rabat, Morocco, Email: [email protected]
Dates: Submitted: 15 April 2019; Approved: 25 April 2019; Published: 26 April 2019
How to cite this article: Hady L, Nassar I, Znati K, Kabbaj N. An uncommon cause of isolated ascites: Pseudomyxoma peritonei. Ann Clin Gastroenterol Hepatol. 2019; 3: 001-005. DOI: 10.29328/journal.acgh.1001007
Copyright License: © 2019 Hady L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ascites; Appendix mucocele; Pseudomyxoma peritonei
Summary
Pseudomyxoma peritonei or Gelatinous Peritoneal Disease is a rare disease. We report a case treated in the department of Hepato-Gastroenterology at Ibn Sina Hospital in Rabat, of a 64-year-old male who presented with an abdominal pain and an increased volume of the abdomen corresponding to ascites. Imaging and anatomopathological study made it possible to diagnose the disease. However, given the general state of the patient, he is under palliative care.
Introduction
Pseudomyxoma peritonei (PMP) or Gelatinous Peritoneal Disease is a rare condition that refers to an anatomo-clinical entity characterized by ascites of variable abundance in the peritoneal cavity, viscous or mucinous, associated or not with neoplastic epithelial cells. It predominates in women. Diagnosis is guided by imaging and confirmed by histology. Prognosis is good in case of early management. We report the case of a male diagnosed with Pseudomyxoma peritonei revealed by isolated ascites.
Case Report
A 64-year-old patient with a history of hypertension treated with amlodipine presented 4 months ago with a progressive abdominal distension and a diffuse abdominal pain predominating in the right iliac fossa, with no associated transit disorders or externalized digestive bleeding, all evolving in a context of impaired general condition with weight loss, anorexia and profound fatigue.
Clinical examination found an altered patient with a WHO performance status at 3, afebrile, anicteric with moderate ascites, with no evidence of portal hypertension or omental cakes, no palpable mass, ganglionic areas were free; the rest of the clinical examination was within normal limits.
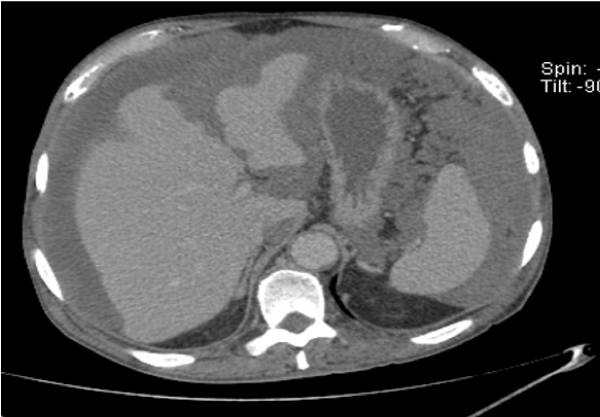
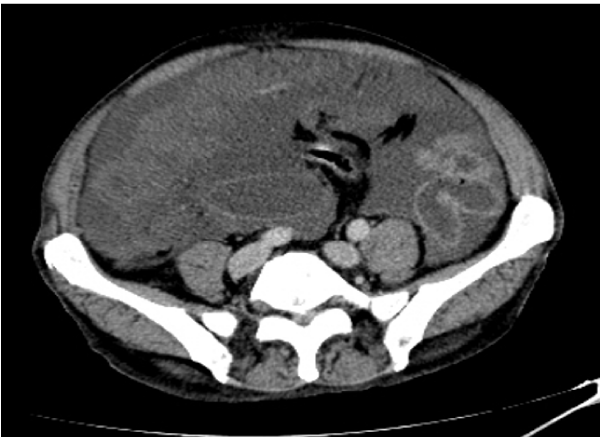
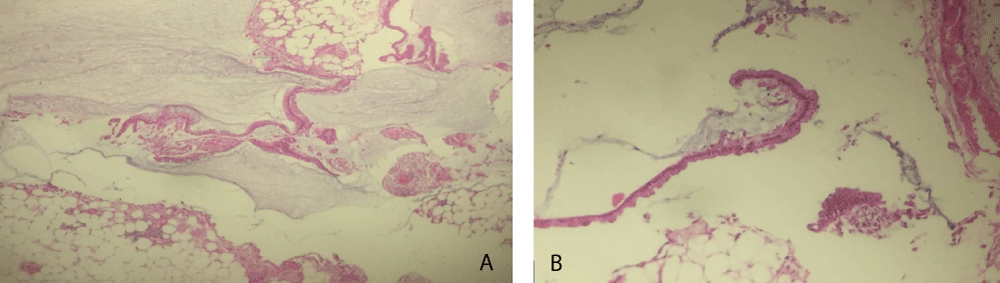
The exploratory puncture of the ascites fluid hardly brought back 15cc of a yellow and viscous liquid of which the biochemical and cytological analysis showed an elevated protein content at 60g / l and a cellularity of 160 leukocytes / mm3 predominantly lymphocyte (69%). Biologically there was a normocytic normochromic anemia (hemoglobin level at 11.9g / dl), and a CRP level elevated to 117.3mg / l; liver and renal functions were normal. Abdominal ultrasound showed a normal-looking liver with free intraperitoneal effusion of great abundance. Chest X-ray was normal. Abdominopelvic computed tomography (CT) showed a high-density abdominopelvic effusion with a nodular peritoneal thickening exerting a mass effect on the liver (liver scalloping Figure 1) associated with a peripheral calcified mass of the right iliac fossa communicating with the coecum evoking an appendicular mucocele (Figure 2) complicated by a Pseudomyxoma peritonei. A laparoscopic peritoneal biopsy showed an adipose tissue containing numerous mucus pools with shreds of uni-stratified mucosal cells and no cytonuclear atypia suggestive of low grade Pseudomyxoma peritonei (Figure 3A,B). The case of the patient was discussed in a multidisciplinary team meeting and given the altered general condition of the patient (WHO performance status 3), a palliative care decision was retained.
Figure 1: Axial CT scan with contrast injection showing abundant ascites with “scalloping” of the liver.
Figure 3: A) Low-grade PMP consisting of mucus puddles containing epithelial structures of glandular and cerebral architecture (H&E stain *10). B) Low grade PMP, mucinous epithelial structures showing minimal cellular atypia (H&E stain *40).
Discussion
Pseudomyxoma peritonei is a clinico-pathological entity corresponding to the intraperitoneal dissemination of mucus-secreting cells responsible for mucin accumulation in the peritoneal cavity. It is a rare disease that was first described in 1842 by Karel Rokitansky then in 1884 by Werth who gave it its current name. Its incidence has recently been estimated at 1 to 2 cases per million inhabitants per year; according to the studies [1], this incidence is 2 per 10,000 laparotomies [2]. Women appear to be more affected than men, with a sex ratio of 1.2 to 3.3 and an age of diagnosis ranging from 20 to 80 years. The case we report concerns a 64-year-old male.
The appendix is now held as the first organ responsible, although mucinous carcinomatosis may occasionally arise from mucinous adenocarcinoma of other organs: gallbladder, stomach, fallopian tubes, ovaries or a colo-rectal origin. In the French multicenter study published in 2010 and involving 301 patients, the origin of Pseudomyxoma peritonei was appendicular in 91% of cases, ovarian in 7% and unknown in 2% of cases [3]. In our patient, the origin is appendicular.
The main pathophysiological pattern used to explain the occurrence of Pseudomyxoma peritonei is that of an appendicular rupture, including epithelial mucinous proliferation, causing mucus and neoplastic swarming in the peritoneal cavity.
Symptomatology is made of a pseudo-appendicular syndrome, intestinal disorders and an abdominal distension as illustrated by our case. This clinical symptomatology is not very specific. In a review of 217 cases of histologically confirmed PMP, 27% had an acute appendicitis presentation, 23% had an abdominal distension as in our patient, 14% had a hernia mostly inguinal, and an isolated abdominopelvic pain in 4% of cases [4]. In women, an ovarian mass can be revealing in 20 to 39% of cases [5,6].
When it comes to imaging, abdominal and pelvic CT with contrast injection and oral markup is the gold standard [6,7]. It can guide the diagnosis when the disease has advanced, by objectifying a festooning (scalloping) of the liver and spleen, which is practically pathognomonic of Pseudomyxoma peritonei, associated to an abundant gelatinous ascites, a bilateral invasion of paracolic gutters, an omental cake and an isolation of the small intestine in the center of the peritoneal cavity [8]. In our patient’s case, CT scan allowed to bring up the diagnosis by showing a major infiltration of the peritoneum and a notched liver.
Among other imaging exams, PET-CT (positron emission tomography-computed tomography) is useful to guide the therapeutic management by evaluating the extension of the disease [9]. The usefulness of serum tumor markers is not well demonstrated, dosage of CA19-9 as a prognostic marker of relapse in treated patients has been suggested by several studies [10,11].
Definitive diagnosis is anatomopathological, based on a puncture of the gelatinous ascites fluid or peritoneal biopsies under laparoscopy. The 2010 WHO classification identifies two types of PMP: low-grade PMPs characterized by the presence of mucin pools with mucus-secreting epithelial cells, uni-stratified epithelial cell shreds and rare mitosis; and high-grade PMPs characterized by an often-high cellularity in mucin pools associated with mucinous adenocarcinoma with an invasive appearance, high grade dysplasia and more frequent mitosis [11]. Our patient had a low-grade PMP.
In the immunohistochemical and molecular study, tumors of appendicular origin express Muc2 and Muc5a, CK20, CDX2 and ACE and are negative for CK7 and CA125. This immunolabeling is useful to determine the site of the primary tumor [12].
Since 1990 and up to the present day, the gold standard in the management of this disease has been based on the combination of a complete cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) [13], which allows to obtain a 5- and 10-year overall survival of 90% and 85%, respectively [14,15]. However, it exposes the patient to a high risk of mortality and postoperative morbidity ranging respectively from 0 to 18% and from 16 to 65%, with different rates depending on the experience of the teams. This heavy treatment can only be offered to well selected patients in a good general condition.
According to the largest series conducted by the Peritoneal Surface Oncology Group (PSOG) on 2298 patients, the 5-year survival rate reached 59%. In case of incomplete cytoreduction, the 5-year survival rate drops to 24% compared to 85% for complete cytoreductions. In addition to that, several studies have highlighted the impact of the grade of PMP on the efficacy of the treatment combining complete cytoreductive surgery and HIPEC. Patients with low grade lesions have a higher survival rate than patients with high grade lesions [16]. With a follow-up of two months, our patient is still alive under palliative treatment.
Conclusion
Pseudomyxoma peritonei is a rare entity, of appendicular origin in the majority of cases. Its clinical symptomatology is nonspecific, diagnosis is brought up by imaging and confirmed by histology. Treatment of choice is the combination of complete cytoreduction and Hyperthermic Intraperitoneal Chemotherapy. Prognosis is related to the early management of the disease, the histological grade and the quality of the surgical procedure.
References
- Shih IM, Yan H, Speyrer D, Shmookler BM, Sugarbaker PH, et al. Molecular genetic analysis of appendiceal mucinous adenomas in identical twins, including one with pseudomyxoma peritonei. Am J Surg Pathol. 2001; 25: 1095-1099. Ref.: https://tinyurl.com/y2ks76zw
- Smeenk RM, van Velthuysen ML, Verwaal VJ, Zœtmulder FA. Appendiceal neoplasms and pseudomyxoma peritonei: a popu- lation based study. Eur J Surg Oncol. 2008; 4: 196-201. Ref.: https://tinyurl.com/y5x4qr2h
- Elias D, Gilly F, Quenet F, Bereder JM, Sidéris L, et al. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperi- toneal chemotherapy. Eur J Surg Oncol. 2010; 36: 456-462. Ref.: https://tinyurl.com/yy3w6cl6
- Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am. 2003; 12: 585–603. Ref.: https://tinyurl.com/y5z72s2j
- Esquivel J. Sugarbaker PH. Clinical presentation of pseudomyxoma peritonei syndrome. Br J Surg. 2000; 87: 1414–1418. Ref.: https://tinyurl.com/y48vnspq
- Smeenk RM, Bruin SC, van Velthuysen ML, Verwaal VJ. Pseudomyxoma peritonei. Curr Probl Surg. 2008; 45: 527-575. Ref.: https://tinyurl.com/y4yskvch
- Fallis SA, Moran BJ. Management of pseudomyxoma peritonei. J BUON. 2015; 20: S47–S55. Ref.: https://tinyurl.com/y2pmyarq
- Sulkin TV, O’Neill H, Amin AI, Moran B. CT in pseudomyxoma peritonei: a review of 17 cases. Clin Radiol. 2002; 57: 608-613. Ref.: https://tinyurl.com/y38dv33k
- Passot G, Glehen O, Pellet O, Isaac S, Tychyj C, et al. Pseudomyxoma peritonei: role of 18F-FDG PET in preo- perative evaluation of pathological grade and potential for complete cytoreduction. Eur J Surg Oncol. 2010; 36: 315-323. Ref.: https://tinyurl.com/yxscjfw7
- Koh JL, Liauw W, Chua T, Morris DL. Carbohydrate anti- gen 19-9 (CA 19-9) is an independent prognostic indicator in pseudomyxoma peritonei post cytoreductive surgery and per- ioperative intraperitoneal chemotherapy. J Gastrointest Oncol. 2013; 4: 173-181. Ref.: https://tinyurl.com/y52ahtms
- Carmignani CP, Hampton R, Sugarbaker CE, Chang D, Sugarbaker PH. Utility of CEA and CA 19-9 tumor markers in diagnosis and prognostic assesment of mucinous epithelial cancer of the appendix. J Surg Oncol. 2004; 87: 162-166. Ref.: https://tinyurl.com/y5aaxwrt
- Dartigues P, Isaac S, Villeneuve L, Glehen G, Capovilla M, et al. Mise au point sur le pseudo-myxome péritonéal. Aspects anatomopathologiques, et implications thérapeutiques. Ann Pathol. 2014; 34: 14–25. Ref.: https://tinyurl.com/y5beavaw
- Sugarbaker PH. Surgical treatment of peritoneal carcinomatosis: 1988 Du Pont lecture. Can J Surg. 1989; 32: 164-170. Ref.: https://tinyurl.com/yxlz9h6a
- Moran B, Baratti D, Yan TD, Kusamura S, Deraco M. Consensus statement on the loco-regional treatment of appendiceal muci- nous neoplasms with peritoneal dissemination (pseudomyxoma peritonei). J Surg Oncol. 2008; 98: 277-282. Ref.: https://tinyurl.com/yy5hsfuh
- Yan TD, Bijelic L, Sugarbaker PH. Critical analysis of treatment failure after complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from appendiceal mucinous neoplasms. Ann Surg Oncol. 2007; 14: 2289-2299. Ref.: https://tinyurl.com/y5xowepd
- Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012; 30: 2449-2456. Ref.: https://tinyurl.com/yxftnuzu