More Information
Submitted: September 09, 2022 | Approved: September 22, 2022 | Published: September 23, 2022
How to cite this article: Zampaglione L, Bornand A, Goossens N, Ramer L, Magini G, et al. A case of coexistent acute severe alcoholic and Q fever hepatitis: The useful contribution of repeated liver biopsies. Ann Clin Gastroenterol Hepatol. 2022; 6: 034-038.
DOI: 10.29328/journal.acgh.1001036
Copyright License: © 2022 Zampaglione L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Alcoholic steatohepatitis; Q fever hepatitis; Coxiella Burnetii; Fibrin-ring granuloma; Liver biopsy
Abbreviations: ASH: Alcoholic Steatohepatitis; AST: Aspartate Amino Transferase; ALT: Alanine Amino Transferase; GGT: Gamma Glutamyl Transferase; MELD: Model for End-Stage Liver Disease; WBC: White Blood Cells; CRP: C-Reactive Protein
A case of coexistent acute severe alcoholic and Q fever hepatitis: The useful contribution of repeated liver biopsies
Lucia Zampaglione1, Aurélie Bornand2, Nicolas Goossens1,3, Lucas Ramer1,3, Giulia Magini1,3, Marie Ongaro1, Andreas Cerny4, Laura Rubbia-Brandt2, Jean-Louis Frossard1 and Laurent Spahr1*
1Gastroenterology and Hepatology, University Hospitals of Geneva, Switzerland
2Clinical Pathology, Switzerland
3Transplantation, Faculty of Medicine, University Hospitals of Geneva, Geneva, Switzerland
4Clinica Luganese Moncucco and Epatocentro Ticino, Lugano, Switzerland
*Address for Correspondence: Laurent Spahr, Gastroenterology and Hepatology, Floor P, University Hospitals of Geneva 4, Rue Gabrielle Perret-Gentil, 1211 Geneva 14, Switzerland, Email: [email protected]
Acute Q fever is a worldwide zoonotic infection due to m2 that may be associated with hepatitis. Nonspecific clinical and biological manifestations may accompany liver involvement, including hepatomegaly and elevated liver biological tests. However, the presence of jaundice is rare. Therefore, making a diagnosis of Q fever hepatitis may be difficult in an afebrile patient with jaundice of recent onset, altered liver function tests, excessive alcohol intake and no reported contact with animals. We report here the diagnostic work-up and complex clinical management of a patient presenting with acute hepatitis resulting from both m2 infection and severe alcoholic steatohepatitis. Positive serology together with a detailed examination of the liver biopsy was able to reveal the coexistence of both Q fever hepatitis with typical fibrin-ring granulomas as well as florid lesions of alcoholic steatohepatitis. A combination of antibiotics, hydroxychloroquine and steroids, guided by the helpful description of changes in histological alterations on repeated liver biopsies during the course of the disease contributed to the slow but favorable outcome.
Q fever is a zoonotic infectious disease caused by the intracellular localization of Coxiella Burnetii, a gram-negative bacteria [1]. Farm and domesticated animals are the typical reservoirs of the infection, with inhalation of contaminated dust being the main route of infections for humans. The clinical presentation of acute Q fever is variable, as approximately 50% of infected individuals remain asymptomatic and a minority demonstrates either non-specific flu-like symptoms or rarely pneumonia or hepatitis [1,2]. Hepatic alterations due to m2 may be associated with increased transaminases and alkaline phosphatase serum levels, although hyperbilirubinemia is a rare observation suggesting a severe liver disease [3,4]. The diagnosis of Q fever hepatitis is based on a positive serological test [1,5] and sometimes a liver biopsy is useful [6,7] in the early phase of infection prior to specific antibody production.
The indication to perform a liver biopsy when a severe form of alcoholic hepatitis is suspected on clinical and biological grounds is still a matter of debate [8]. However, histological demonstration of an alternative diagnosis (in up to approximately 20% of suspected cases [9,10] avoids the inappropriate and potentially hazardous administration of corticosteroids in patients with advanced liver failure and a high risk of infections [11].
We present the case of a patient admitted to our hospital with severe alcoholic hepatitis in whom a diagnosis of concomitant Q fever hepatitis was made driven by liver histology and serology. The complex management of these two causes of acute liver injury comprised of a combination of doxycycline, hydroxychloroquine and steroids guided by repeated liver biopsies allowing for a slow but complete return to normal values.
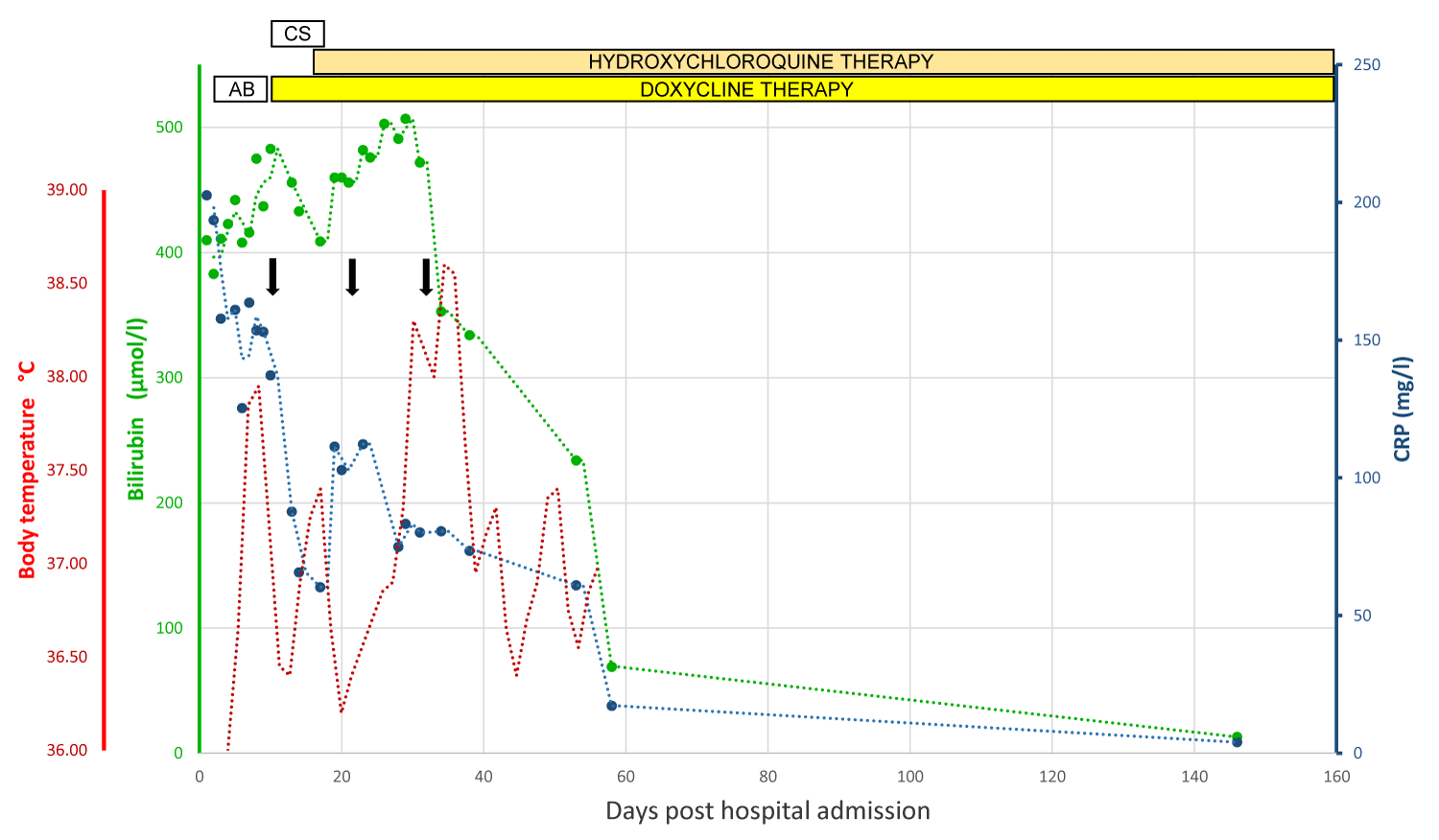
A 65-year-old man was transferred from a regional hospital in Ticino (southern part of Switzerland) to our department on May 26, 2021, for suspicion of severe alcoholic steatohepatitis (ASH). His medical history consisted of type 2 diabetes, arterial hypertension treated with lercanidipine and obesity (BMI 31 kg/m2). One week prior to admission, he presented jaundice, fatigue and mild epigastric discomfort. He declared an alcohol consumption of 60 gr daily, denied any recent travel and was living in a rural area without any contact with farm animals. On physical examination, the patient was alert, oriented and afebrile. He had marked jaundice, pitting malleolar edema, several spider naevi on the thorax and no flapping tremor. Blood tests revealed mild normocytic anemia (hemoglobin 115 gr/L), normal platelet count and leukocytosis (white blood cells (WBC) 13.7 G/L). Coagulation tests were altered with a prothrombin time at 15%, INR 3.8, and factor V level of 52%. Liver function tests were as follows: AST 73 IU/L [N:<50], ALT 76 IU/L [N:<50]), alkaline phosphatase 170 IU/L [N: <102], gamma-glutamyl -transferase (GGT) 418 IU/L [N.<40], and total serum bilirubin 410 umol/L [N:<25] with a conjugated bilirubin of 365 umol/L. Serum values of creatinine were normal, but albumin was decreased at 22 gr/L [N:>35] and C-reactive protein (CRP) was increased at 202 mg/L [N:<10]. Urine, stool and blood cultures returned negative. Thoraco-abdominal imaging by CT scan showed mild bilateral pleural effusion, no pneumonia, dysmorphic liver with steatotic parenchyma, moderate ascites and no vascular thrombosis. At cholangiography by MR imaging bile ducts were normal. Additional blood tests excluded viral hepatitis A, B, C and E and showed normal IgG levels and the absence of anti-actin antibodies. Ceruloplasmin and a-1-antitrypsin levels were within the normal range. On day 4 of hospitalization, the patient developed a high fever (T° 38.4 °C) with a high WBC count (25 G/L) and elevated pro-calcitonin (2 mg/L [N:<0.5], all of which justified the empirical administration of broad-spectrum antibiotic therapy combining piperacillin and tazobactam. A transjugular liver biopsy performed on day 5 demonstrated clinically significant portal hypertension (hepatic venous pressure gradient: 14 mmHg [N<5 mmHg]). The biopsy specimen was formalin-fixed, paraffin-embedded, and then stained using haematoxylin and eosin (H&E), Masson’s Trichrome, Gomori, Prussian Blue reaction and PAS-Diastase. Microscopic examination under 200-400x magnification showed extensive liver fibrosis and histological criteria for a diagnosis of severe steatohepatitis with cholestasis, as well as several fibrin-ring granulomas present throughout the entire biopsy specimen. These granulomas are characterized by a central optically clear vacuole surrounded by fibrin and leucocytes also known as « doughnut granuloma » [12-14]. This granulomatous inflammation in the setting of febrile illness prompted us to complete the diagnostic workup with additional serology testing for brucellosis, tuberculosis, tularemia, leptospirosis, and Q fever. Serologic testing for Coxiella Burnetii by means of immunofluorescence assay returned strongly positive (phase II IgG antibody titer, 1/163840 [+ if >1/40] and phase II IgM antibody titer, 1/320 [+ if >1/40]). Bone marrow biopsy was therefore not considered necessary for the diagnosis [14]. Thus, a diagnosis of severe alcoholic hepatitis (Maddrey’s score 82, MELD score 26) and concomitant granulomatous hepatitis due to m2 was made. Piperacillin-tazobactam was stopped and doxycyclin 200 mg per day was administered intravenously. Steroid therapy with prednisone 40 mg daily was initiated 72 hours later but stopped 7 days later due to nonresponse according to a Lille score of 0.8 [15]. A heart murmur in the aortic area at auscultation as well as an echocardiogram revealing vegetations on the aortic valve led to a suspicion of Q fever endocarditis and oral hydroxychloroquine 600 mg daily was added to the antimicrobial therapy. The patient continued to present intermittent bouts of fever (peaks of 38.7 °C) until day 26 of hospitalization, C-reactive protein levels remained elevated and serum bilirubin started to decline slowly around day 30.
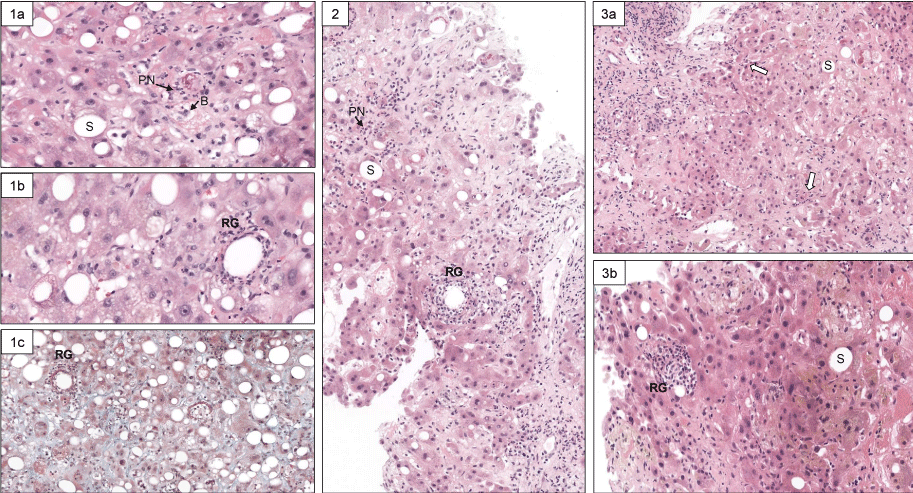
We were facing the difficult management of two distinct acute inflammatory liver diseases in a patient with persistent poor liver function (MELD score between 23 and 30) receiving antibiotics but no anti-inflammatory treatment. Therefore, we decided to repeat liver biopsies during the hospital stay in order to assess the changes in histological lesions of Q fever hepatitis, and alcoholic steatohepatitis. Thus, histological examination of the 3 sequential liver biopsy specimens obtained by a transjugular route at baseline, day 22 and day 36 of hospitalization showed an overall progressive decline in the severity of lesions (Figure 1 and Table 1).
Figure 1: Histological view of repeated liver biopsies (H & E stain) at baseline (Panel 1), on day 22 (Panel 2), and day 36 (Panel 3) of hospitalization. At baseline, typical lesions of steatohepatitis are present (Panel 1a, original magnification x 200: PN: polynuclear neutrophils; S: steatosis; B: hepatocyte ballooning) and characteristic granulomas (panel 1b, original magnification x 400: RG: fibrin-ring granuloma) (Panel 1c: Landrum’s stain for fibrin coloration, original magnification x 200). The second liver biopsy (Panel 2) showed a reduction of steatosis and less RG (original magnification x 200). On the third liver biopsy, a mild residual PN inflammation persists (open arrows) and S is minimal (Panel 3a). A remnant of RG is visible (H&E, original magnification x 400).
| Table 1 | ||||
| Histological lesion | Biopsy #1 | Biopsy #2 | Biopsy #3 | |
| Alcoholic steatohepatitis | Steatosis | 1 | 0 | 0 |
| Inflammation | 1 | 1 | 0 | |
| Ballooning | 1 | 1 | 1 | |
| Mallory-Denk bodies | 1 | 1 | 1 | |
| Fibrosis stage | 2 | 2 | 2 | |
| Q Fever hepatitis | Fibrin-ring granuloma | +++ | ++ | + |
| Note: Semi-quantitative evaluation of severity of histological lesions in repeated transjugular liver biopsies focused on ASH and Q fever hepatitis. For ASH, the score was as followed, adapted from ref [21]: steatosis: [< 33% = 0; 33% - 66% = 1; > 66% = 2]. Polynuclear inflammation: [mild: = 0; moderate/severe: = 1]. Ballooning: [mild: = 0; marked: = 1]. Fibrosis: [absent or periportal = 0; expansive periportal = 1; extensive fibrosis/cirrhosis = 2]. Mallory-Denk bodies: [absent: = 0; present = 1]. For Q fever hepatitis: Fibrin-ring granuloma: +++ = numerous; ++ : few: + = scarce |
||||
As the patient’s clinical condition was slowly improving without recurrent fever or cardiac decompensation, and with a consistent decline in serum bilirubin and improvement in coagulation function, the patient was discharged with a daily combination of 200 mg doxycyclin and 600 mg hydroxychloroquine to be continued for a total of 12 to 18 months according to patient’s tolerance. Serum bilirubin had returned to normal value on day 148 after hospital admission. He was also strongly advised to maintain absolute and durable alcohol abstinence as a key prognostic factor [8,16]. The patient’s clinical course is illustrated in Figure 2.
Figure 2: Graphical illustration of the clinical course of the patient’s liver injury. Total bilirubin appears in the green line, CRP in the blue line, and body temperature in the red line. The vertical black arrow indicates liver biopsy. Abbreviations: CRP: C-Reactive Protein; AB: Broad-Spectrum Antibiotics; CS: Corticosteroids.
Q fever is a widespread infectious disease due to Coxiella Burnetii, an intracellular Gram-negative pathogen that may give rise to acute manifestations including a « flu-like » illness, pneumonia, and hepatitis, but also a chronic disease such as endocarditis [1]. The infection may also be asymptomatic as reported in a Swiss alpine valley outbreak in which half of the infected individuals became ill [17]. Humans are typically infected by breathing aerosols or ingesting food contaminated by m2 in a rural environment with domesticated animals. However, a clear history of exposure is not always identified [1,18], as in our case. The clinical presentation and severity of the disease may be influenced by the virulence of the pathogen strain, but also by host factors. Infection by m2 is more frequently reported in males than in females (although this may be related to different occupational exposure) and conditions affecting the host’s immune system have been reported to either prolong the course of the disease [2] or aggravate the patient’s condition [19]. This statement is supported by the severe illness and liver insufficiency in our patient with concomitant acute Q fever and severe ASH with extensive liver fibrosis. Liver involvement in acute Q fever is variable, presenting with only a mild elevation of liver function tests in most studies [18], while acute severe hepatitis with jaundice is relatively rare [3,4].
Making a diagnosis of acute Q fever with hepatitis may not be straightforward in the absence of a history of exposure to cattle or other domesticated animals. On the one hand, fever, fatigue, vague abdominal discomfort and moderately elevated liver function tests are non-specific symptoms compatible with a number of viral infections [3]. On the other hand, clinical symptoms including fever and jaundice are described in the syndrome of alcoholic hepatitis making biliary tract disease and drug-induced liver injury part of the differential diagnoses [8]. At a young age, low white blood cell count and high CRP may differentiate acute Q fever from other infections [5]. However, a definite diagnosis is based on serology using an immunofluorescence assay, with detectable IgG and IgM antiphase II antibodies in serum in a compatible clinical context. The role of liver histology in the diagnosis of Q fever hepatitis is controversial. Liver biopsy findings are non-specific, although the presence of fibrin ring granulomas is a characteristic feature [12]. In our case, liver biopsy was able to determine the coexistence of both steatohepatitis lesions (steatosis, polynuclear rich inflammation, ballooned hepatocytes) and histological alterations that were highly suggestive of acute C. burnetii infection [2,7]. This was a clinically relevant observation with regards to the indication for corticosteroids in severe ASH, as relying on clinical and biological criteria alone may lead to misclassification and the inappropriate use of steroids with potentially adverse consequences [9,10]. In the present case, the very elevated serum level of CRP was a finding incongruent with suspicion of ASH, suggesting the coexistence of an acute infection.
Combined pharmacological therapy of prednisone, doxycycline and hydroxychloroquine was used resulting in a slow but sustained improvement of the patient’s condition. The patient was initially declared as a steroid non-responder for ASH and we decided not to pursue corticotherapy although lines of evidence suggest this treatment may be effective in the treatment of C. burnetii granulomatous hepatitis [20,21].
This clinical case of severe hepatitis due to both acute Q fever infection and alcoholic steatohepatitis underlines the importance of considering alcoholic hepatitis as a syndrome characterized by new-onset jaundice and signs of hepatic insufficiency in the setting of heavy alcohol use [8]. Considering acute infections as alternative diagnoses allowed us to determine that both ASH and acute Q fever infections were indeed responsible for the patient’s condition. This case also underlined the key role of liver histology as a major tool to establish the diagnosis and guide clinical management in this complex situation.
Funding statement
The present work didn’t require specific funding and was performed as part of the employment of the authors at the University Hospitals of Geneva, Switzerland.
- Parker NR, Barralet JH, Bell AM. Q fever. Lancet. 2006 Feb 25;367(9511):679-88. doi: 10.1016/S0140-6736(06)68266-4. PMID: 16503466.
- Geha R, Peters M, Gill RM, Dhaliwal G. Histology Rings True. N Engl J Med. 2017 Mar 2;376(9):869-874. doi: 10.1056/NEJMcps1609391. PMID: 28249146.
- Ali S, Prakash S, Murali AR. Hepatic Manifestations of Nonhepatotropic Infectious Agents Including Severe Acute Respiratory Syndrome Coronavirus-2, Adenovirus, Herpes Simplex Virus, and Coxiella Burnetii. Gastroenterol Clin North Am. 2021 Jun;50(2):383-402. doi: 10.1016/j.gtc.2021.02.012. Epub 2021 Apr 23. PMID: 34024447.
- Choi HC, Lee SH, Kim J, Kim SH, Hwang JH, Kim JW, Jeong SH, Kim H. A case of acute q Fever with severe acute cholestatic hepatitis. Gut Liver. 2009 Jun;3(2):141-4. doi: 10.5009/gnl.2009.3.2.141. Epub 2009 Jun 30. PMID: 20431739; PMCID: PMC2852695.
- Keijmel SP, Krijger E, Delsing CE, Sprong T, Nabuurs-Franssen MH, Bleeker-Rovers CP. Differentiation of Acute Q Fever from Other Infections in Patients Presenting to Hospitals, the Netherlands. Emerg Infect Dis. 2015 Aug;21(8):1348-56. doi: 10.3201/eid2108.140196. PMID: 26196955; PMCID: PMC4517711.
- Vanden Bussche S, Smets K, Steelandt T, Van Eyken P, Caenepeel P, Robaeys G. A case of Q fever with hepatitis and an atypical skin lesion. Acta Gastroenterol Belg. 2018 Jul-Sep;81(3):441-442. PMID: 30350538.
- Lee M, Jang JJ, Kim YS, Lee SO, Choi SH, Kim SH, Yu E. Clinicopathologic features of q Fever patients with acute hepatitis. Korean J Pathol. 2012 Feb;46(1):10-4. doi: 10.4132/KoreanJPathol.2012.46.1.10. Epub 2012 Feb 23. PMID: 23109972; PMCID: PMC3479695.
- European Association for the Study of the Liver. Electronic address: [email protected]; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018 Jul;69(1):154-181. doi: 10.1016/j.jhep.2018.03.018. Epub 2018 Apr 5. PMID: 29628280.
- Ramond MJ, Poynard T, Rueff B, Mathurin P, Théodore C, Chaput JC, Benhamou JP. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992 Feb 20;326(8):507-12. doi: 10.1056/NEJM199202203260802. PMID: 1531090.
- Spahr L, Lanthier N, Tihy M, Frossard JL, Rubbia-Brandt L, Goossens N. Clinical Presentation and Gene Expression of Acute Alcohol-Induced Microvesicular Steatosis Mimicking Alcoholic Hepatitis. Hepatol Commun. 2021 Jan 9;5(4):618-628. doi: 10.1002/hep4.1669. PMID: 33860120; PMCID: PMC8034579.
- Gustot T, Felleiter P, Pickkers P, Sakr Y, Rello J, Velissaris D, Pierrakos C, Taccone FS, Sevcik P, Moreno C, Vincent JL; EPIC II Group of Investigators. Impact of infection on the prognosis of critically ill cirrhotic patients: results from a large worldwide study. Liver Int. 2014 Nov;34(10):1496-503. doi: 10.1111/liv.12520. Epub 2014 Mar 26. PMID: 24606193.
- Dauby N, Gomez Galdon M, Montesinos I, Van Esbroeck M, Sersté T. Q-fever associated granulomatous hepatitis. Int J Infect Dis. 2020 Jun;95:113-114. doi: 10.1016/j.ijid.2020.04.002. Epub 2020 Apr 10. PMID: 32283284.
- Aguilar-Olivos N, del Carmen Manzano-Robleda M, Gutiérrez-Grobe Y, Chablé-Montero F, Albores-Saavedra J, López-Méndez E. Granulomatous hepatitis caused by Q fever: a differential diagnosis of fever of unknown origin. Ann Hepatol. 2013 Jan-Feb;12(1):138-41. PMID: 23293205.
- Travis LB, Travis WD, Li CY, Pierre RV. Q fever. A clinicopathologic study of five cases. Arch Pathol Lab Med. 1986 Nov;110(11):1017-20. PMID: 3778120.
- Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, Dharancy S, Texier F, Hollebecque A, Serfaty L, Boleslawski E, Deltenre P, Canva V, Pruvot FR, Mathurin P. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007 Jun;45(6):1348-54. doi: 10.1002/hep.21607. PMID: 17518367.
- Louvet A, Labreuche J, Artru F, Bouthors A, Saffers P, Rolland B, Dharancy S, Lassailly G, Canva-Delcambre V, Duhamel A: Drivers of short- and long-term mortality in severe alcoholic hepatitis: a complex relationship between alcohol relapse and early improvement in liver function. Hepatology 2016 ; 64:22a-22a.
- Dupuis G, Petite J, Péter O, Vouilloz M. An important outbreak of human Q fever in a Swiss Alpine valley. Int J Epidemiol. 1987 Jun;16(2):282-7. doi: 10.1093/ije/16.2.282. PMID: 3301708.
- Finn T, Babushkin F, Geller K, Alexander H, Paikin S, Lellouche J, Atiya-Nasagi Y, Cohen R. Epidemiological, clinical and laboratory features of acute Q fever in a cohort of hospitalized patients in a regional hospital, Israel, 2012-2018. PLoS Negl Trop Dis. 2021 Jul 15;15(7):e0009573. doi: 10.1371/journal.pntd.0009573. PMID: 34264953; PMCID: PMC8315502.
- Raoult D. Host factors in the severity of Q fever. Ann N Y Acad Sci. 1990;590:33-8. doi: 10.1111/j.1749-6632.1990.tb42204.x. PMID: 2198833.
- Crespo M, Sopeña B, Bordón J, de la Fuente J, Rubianes M, Martinez-Vázquez C. Steroids treatment of granulomatous hepatitis complicating Coxiella Burnetii acute infection. Infection. 1999 Mar-Apr;27(2):132-3. doi: 10.1007/BF02560514. PMID: 10219646.
- Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, Augustin S, Mookerjee RP, Michelena J, Smyrk TC, Buob D, Leteurtre E, Rincón D, Ruiz P, García-Pagán JC, Guerrero-Marquez C, Jones PD, Barritt AS 4th, Arroyo V, Bruguera M, Bañares R, Ginès P, Caballería J, Roskams T, Nevens F, Jalan R, Mathurin P, Shah VH, Bataller R. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014 May;146(5):1231-9.e1-6. doi: 10.1053/j.gastro.2014.01.018. Epub 2014 Jan 15. PMID: 24440674; PMCID: PMC3992184.