More Information
Submitted: July 15, 2024 | Approved: July 24, 2024 | Published: July 25, 2024
How to cite this article: Patel M, Patel A, Kshatriya P. Mesenchymal Stem Cell Therapy for Hepatic Encephalopathy Due to Advance Liver Cirrhosis: Case Study. Ann Clin Gastroenterol Hepatol. 2024; 8(1): 017-020. Available from: https://dx.doi.org/10.29328/journal.acgh.1001046.
DOI: 10.29328/journal.acgh.1001046
Copyright License: © 2024 Patel M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hepatic encephalopathy, end-stage liver disease, liver cirrhosis, mesenchymal Stem cell
Mesenchymal Stem Cell Therapy for Hepatic Encephalopathy Due to Advance Liver Cirrhosis: Case Study
Megha Patel, Ankur Patel and Prashant Kshatriya*
Senior Scientist at Viecell Institute of Regenerative Medicine, Surat, Gujarat, India
*Address for Correspondence: Prashant Kshatriya, Senior Scientist, Viecell Institute of Regenerative Medicine, Surat, Gujarat, India, Email: [email protected]
Mesenchymal stem cell (MSC) transplantation may be an alternative to liver transplantation for patients with end-stage liver disease. A 24-year-old patient with Hepatic Encephalopathy due to alcoholic liver cirrhosis underwent UCMSC transplantation because there were no donors available for liver transplants involving adult deceased and living individuals. The patient was given allogeneic Umbilical cord-derived MSCs, which were then cultured following accepted practices. Subsequently, the UCMSCs were infused through the intravenous route 3 times at the interval of 30 days. Serum bilirubin, globulin, and ammonia levels were improved after the infusion and the morphology of the liver and spleen had also improved.
One impact of neurotoxins that enter the bloodstream and travel to the brain is hepatic encephalopathy (HE)[1]. Hepatic encephalopathy (HE) can be caused by several variables, including inflammation, toxins, necrotic liver, blood ammonia levels, and systemic inflammatory response syndrome (SIRS) [2]. A small amount of ammonia remains in the hepatic vessels under normal circumstances because the liver effectively excretes the ammonia produced by the body through the urea cycle and glutamine synthesis. The liver's capacity to expel ammonia from the hepatic veins is lost in Acute Liver Failure, and the hepatic vein's ammonia content rises. Through glutamine synthesis, the muscles and brain start the detoxification process from ammonia. As a result, these two tissues are regarded as glutamine-releasing and ammonia-scavenging organs [3].
Hyperammonemia is often associated with hepatic encephalopathy (HE) and cirrhosis (LC). Decreased detoxification capacity of the liver caused hyperammonemia [4]. Infections with urease-producing bacteria, high- protein diets, malignancies, sarcopenia, renal failure, gastrointestinal bleeding, gastric bypass, and organ transplantation can all cause high ammonia levels and noncirrhotic encephalopathy [5,6].
Many different organs, including the liver, brain, kidneys, muscles, and gastrointestinal tract, are involved in ammonia metabolism. Therefore, malnourishment, persistent multiorgan dysfunction, and low non-cerebral function reserves may be indicated by hyperammonemia [4]. Several neoteric investigations have demonstrated the link between hyperammonemia and unfavorable outcomes in people with clinically stable severe chronic liver disease or cirrhosis. These outcomes include hospitalization, liver-related repercussions (LRCs), progression to LC, and mortality [6,7]. Previous studies found that ammonia was directly associated with LC-grade, organ failure such as the liver, kidney, and brain, and is an independent risk factor for 28-day death [7,8]. These findings provided evidence that ammonia levels are not only clinically relevant in assessing HE severity but may also be a prognostic biomarker for identifying patients at high risk of poor outcomes..
Due to their low concentration of class I MHC molecules and lack of expression of class II major histocompatibility complex (MHC) antigens, MSCs are almost immune-privileged. Furthermore, MSCs lack the co-stimulatory molecules— such as CD80, CD86, and CD40—that are necessary for immune recognition, which is the foundation for their allogeneic use [9]. As a result, MSCs from both autologous and allogeneic donors have been used in clinical settings.
This study aimed to evaluate the safety and eventual efficacy of umbilical cord- derived mesenchymal stem cell transfusion in patients with hepatic encephalopathy due to advanced liver cirrhosis.
A 24-year-old patient had first reported to our hospital with symptoms of chronic liver disease. He had a history of excessive alcohol consumption and all other etiological markers for liver disease confirmed positive.
At the time of presentation, he had end-stage cirrhosis of the liver, minor ascites, peripheral edema, and jaundice. His serum ammonia level was 419 mg/dL, which is too high other symptoms include forgetfulness, confusion, disorientation, slurred speech, and shaking of arms.
His liver function test parameters are also elevated. The final diagnosis was Hepatic Encephalopathy due to Alcoholic Liver Disease (ALD). After the initial presentation, he suffered two episodes of hepatic encephalopathy, which required hospitalization. He was counselled and advised for urgent liver transplantation but due to lack of donor and financial incapability, it was not possible. Due to the deterioration of the patient's clinical condition, we discussed his request for MSC transplantation. After obtaining ethical approval from the institution and informed consent from the patient.
An Umbilical Cord-derived Mesenchymal Stem Cells (UCMSCs) transplantation was conducted in three sittings at the interval of 30 days. The UCMSC isolation was done as per our earlier protocol [12] in which Umbilical Cords (UCs) were used as a source of the allogeneic MSCs. The UCs were used within 2 h of delivery, which were obtained from Rupal Hospital, Surat, Gujarat. The cords were cleaned with 70% alcohol also washed completely with saline and digested mechanically and enzymatically with collagenase I under sterile conditions. The dissolved cord was gathered and suspended in the growth medium. The digested cord was placed in 6-well plates and dressed in Dulbecco's Modified Eagle's Medium (DMEM) (Sigma, Germany), supplemented with 10 (v/v) Fetal/Bovine Serum (FBS) (Sigma, Germany), 2 mM L- glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin and 1 μg/mL amphotericin B (Lonza, Belgium). The culture plate was placed in an incubator with impregnated moisture at 5% CO2 at 37 ℃. The medium was changed doubly weekly till 80% confluency was reached. The cells were detached with trypsin, counted and seeded in a vented tissue culture flask (Corning, USA) until passage 3. Eventually, the cells were detached, counted, washed, and suspended in the normal saline for intravenous infusion.
The MSC transplantation procedure involved injecting the cells directly into the bloodstream. During the initial procedure, 80 million MSCs were injected. Subsequently, the transplantation was repeated after 30 days, followed by another transplantation after an additional 30 days, during which an extra 80 million MSC stem cells were injected. Following the initial, second, and third MSC transplantations, the patient received outpatient follow-up care.
After initial MSC transplantation, there appeared to be an improvement in serum ammonia, and liver function test parameters and also there are positive changes in the USG report. The ammonia level continuously decreases to a normal level.
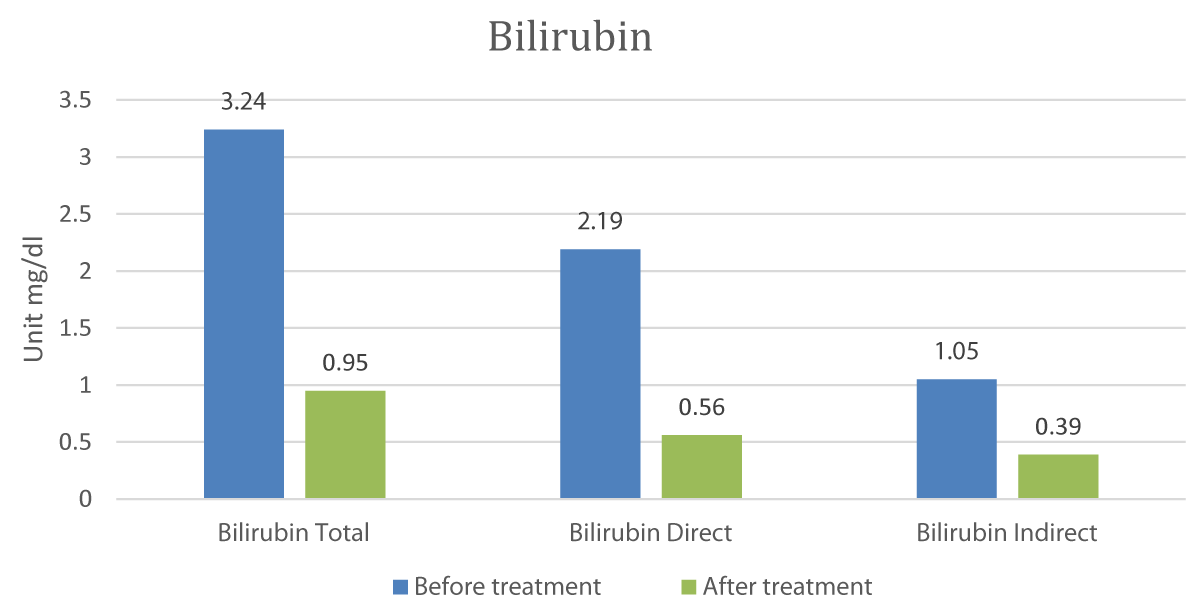
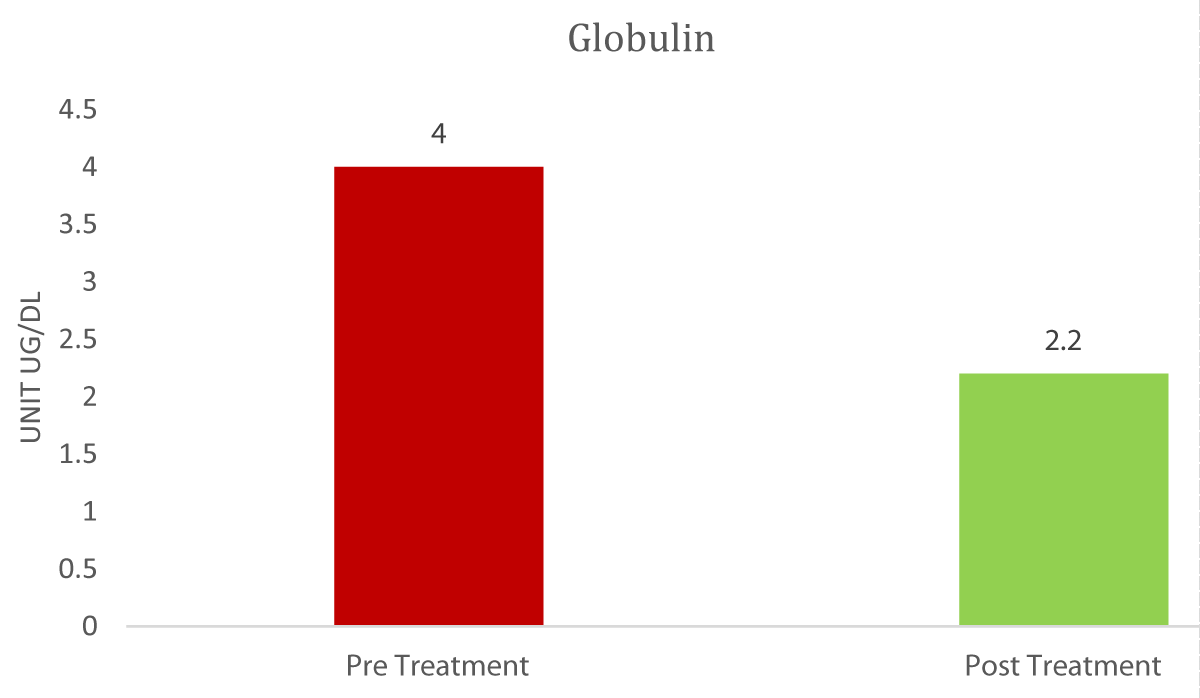
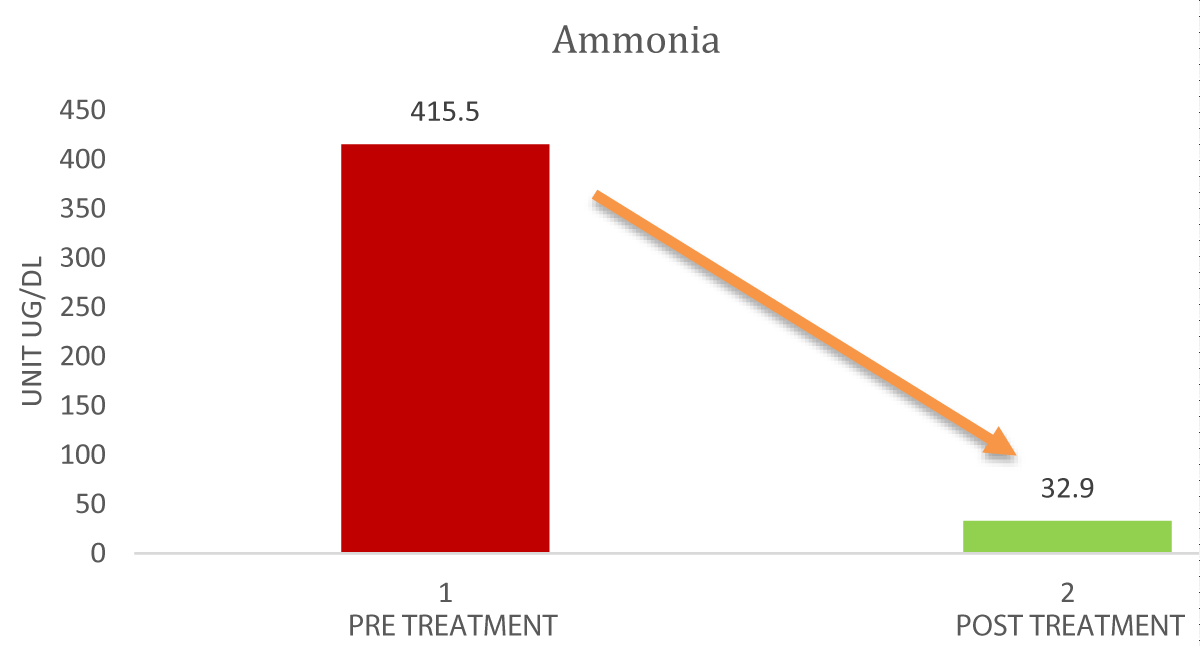
The Graphs 1-3, and Table 1 show the comparison and improvement in the functioning and morphology of the liver after 3 cycles of allogeneic transplantation of Mesenchymal Stem Cells (MSCs). Graph 1 represents the reduction in the total bilirubin, direct bilirubin, and indirect bilirubin. Graph 2 shows the reduction in the globulin level and Graph 3 indicates the abatement of ammonia.
Graph 1: Comparison of Bilirubin Pre and post-treatment.
Graph 2: Comparison of Globulin Pre and post-treatment.
Graph 3: Comparison of Ammonia Pre and post-treatment.
Table 1 shows that the liver and spleen have improved morphologically after treatment in terms of size and texture.
| Table 1: Pre and Post-treatment USG report comparison. | ||
| USG parameters | Pre-treatment | Post-treatment |
| Liver Size | 17 cm- Hepatomegaly | 15.3 cm- Mild Hepatomegaly |
| Texture | Irregular | Normal |
| Spleen | 13.5 cm- Splenomegaly | 11.5 cm- Normal |
Advanced liver dysfunction can induce hepatic encephalopathy. When neurotoxic substances like ammonia build up in the bloodstream, it manifests as a variety of neuropsychiatric abnormalities. Although it is frequently linked to cirrhosis and other liver diseases, it can also occur in people who have never had a liver problem before. Brain edema and, in extreme situations, coma can result from acute severe liver failure in people without a history of liver illness [10].
Patients with chronic liver disease may be able to reverse and manage their hepatic encephalopathy. However, because of widespread brain swelling and structural brainstem injuries, it becomes more difficult to control when it occurs acutely with rapidly rising blood ammonia levels [10].
There are several ways, stem cells support liver regeneration. The processes that are being proposed include transdifferentiation into hepatocytes, cell fusion between sick hepatocytes and stem cells, division of fused cells to replenish the liver, and stimulation of angiogenesis to facilitate the quick growth of healthy hepatocytes [11,12].
The stem cells can be autologous or allogeneic. If the stem cells are isolated from the patient's own body they are autologous and if stem cells are used from.
Unrelated donor or cell banking is called allogeneic, and both types have their advantages and disadvantages. Depending on the patient's condition the source of stem cells can be decided. For our patient, we decided to proceed with the allogeneic source of stem cells i.e. mesenchymal stem cells from umbilical cord tissue. Several research ongoing studies are confirming the safety and efficacy of mesenchymal stem cells.
MSCs can be transplanted with many different routes. It can be intravenous or directly on the liver through a hepatic artery or portal vein. Seeing the patient's condition we decided to infuse the MSCs through the intravenous route as it is simpler and there is very less risk.
Based on registered clinical trial data on the use of MSCs for liver treatment, the majority of infused cells are BMMSCs or UCMSCs. MSCs can be isolated from multiple human tissues and the homing and regenerative capacity varies depending on the sources [13,14]. Which type of MSCs has the greatest therapeutic efficacy in liver cirrhosis remains an unsolved issue, despite many meta-analyses showing that BM-MSCs outperformed UC-MSCs in therapeutic effects [15].
The majority of animal and human research employing MSCs has demonstrated their therapeutic efficacy. However, research suggests that MSC engraftment is minimal due to their short-term survival upon injection16. One drawback of giving IV infusion of MSCs is that most of the cells get trapped in the lungs and a very small number of cells reach the affected organ. To overcome this problem, injection on the affected site can help get better results [17-19].
Our experience with a UCMSC transplantation case in hepatic encephalopathy caused by end-stage liver cirrhosis suggests that the patient showed some biochemical and clinical improvements following three cycles of UCMSC infusions. The patient was admitted on 31st May 2023 and underwent MSC infusion at the interval of one month each and currently after one year the patient's report has shown a notable improvement in liver function. Currently, the patient is almost fit and continuing his routine work without any medications. However, no immunological marker was assessed, which could be a disadvantage in this case.
In conclusion, our single case study suggests that UCMSC transplantation via intravenous route is safe in the treatment of Hepatic Encephalopathy due to liver cirrhosis and there is a noticeable improvement in the biochemical parameters and USG parameters after the treatment. Therapies involving BMCs, HSCs, hepatocytes, and MSCs are currently being used as an alternative to liver transplantation, which is the principal treatment for end-stage liver disease. MSCs are being studied as a potential cell source for treatments that improve liver function. They travel to the wounded liver tissues, differentiate into hepatocytes, reduce liver inflammation and fibrosis, and exhibit antioxidant activity. However, further research is needed to enhance the regenerative healing properties of MSCs through methods such as MSC-priming, MSC-derived exosomes, genetic modification, and 3D-culture techniques with larger study groups. But while awaiting a liver transplant, MSC infusion can be utilized as a bridge treatment for advanced liver cirrhosis.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed consent
Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. The study is approved by internal IEC VIE/HE/2023.
- Geiger A, Magnes J, Taylor Rm, Veralli M. Effect of blood constituents on uptake of glucose and on metabolic rate of the brain in perfusion experiments. Am J Physiol. 1954;177(1):138-149. Available from: https://doi.org/10.1152/ajplegacy.1954.177.1.138
- Antoniades CG, Berry PA, Wendon JA, Vergani D. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49(5):845-61. Available from: https://doi.org/10.1016/j.jhep.2008.08.009
- Vaquero J, Chung C, Cahill ME, Blei AT. Pathogenesis of hepatic encephalopathy in acute liver failure. Semin Liver Dis. 2003;23(3):259-69. Available from: https://doi.org/10.1055/s-2003-42644
- Wright G, Sharifi Y, Jover-Cobos M, Jalan R. The brain in acute on chronic liver failure. Metab Brain Dis. 2014;29(4):965-73. Available from: https://doi.org/10.1007/s11011-014-9553-0
- Ong JP, Aggarwal A, Krieger D, Easley KA, Karafa MT, Van Lente F, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114(3):188-93. Available from: https://doi.org/10.1016/S0002-9343(02)01477-8.
- Vierling JM. Masoud Mokhtarani, Robert S. Brown Jr., Parvez Mantry, Don C. Rockey, Marwan Ghabril et al. Fasting blood ammonia predicts risk and frequency of hepatic encephalopathy episodes in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2016;14:903-906.e901. https://doi.org/10.1016/j.cgh.2015.11.018
- Hu, C. Kaizhou Huang, Lingfei Zhao, Fen Zhang, Zhongwen Wu & Lanjuan Li, et al. Serum ammonia is a strong prognostic factor for patients with acute-on-chronic liver failure. Sci. Rep. 2020;10: 16970. https://doi.org/10.1038/s41598-020-73603-1
- Shalimar, Sheikh MF, Mookerjee RP, Agarwal B, Acharya SK, Jalan R. Prognostic Role of Ammonia in Patients With Cirrhosis. Hepatology. 2019;70(3):982-994. Available from: https://doi.org/10.1002/hep.30534
- Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318-24. Available from: https://doi.org/10.1002/path.2469
- Patel M, Patel A, Kshatriya P. Subcutaneous and Intravenous Administration of Human Umbilical Cord Mesenchymal Stem Cells in Patient with Multiple Foot Ulcers Complicated by Scleroderma- A Case Report with One Year Follow Up. J Reg Med Biol Res. 2023;4(3):1-6. Available from: https://doi.org/10.46889/JRMBR.2023.4302
- Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375(17):1660-1670. Available from: https://doi.org/10.1056/nejmra1600561
- Moore JK, Stutchfield BM, Forbes SJ. Systematic review: the effects of autologous stem cell therapy for patients with liver disease. Aliment Pharmacol Ther. 2014;39(7):673-685. Available from: https://doi.org/10.1111/apt.12645
- Pan XN, Zheng LQ, Lai XH. Bone marrow-derived mesenchymal stem cell therapy for decompensated liver cirrhosis: a meta-analysis. World J. Gastroenterol. 2014;20:14051-14057. Available from: https://doi.org/10.3748%2Fwjg.v20.i38.14051
- Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl Med. 2018;7(9):651-663. Available from: https://doi.org/10.1002/sctm.18-0024
- Sisakhtnezhad S, Alimoradi E, Akrami H. External factors influencing mesenchymal stem cell fate in vitro. Eur J Cell Biol. 2017;96(1):13-33. Available from: https://doi.org/10.1016/j.ejcb.2016.11.003
- Saleh M, Taher M, Sohrabpour AA, Vaezi AA, Nasiri Toosi M, Kavianpour M, et al. Perspective of placenta derived mesenchymal stem cells in acute liver failure. Cell Biosci. 2020;10:71. Available from: https://doi.org/10.1186%2Fs13578-020-00433-z
- Eom YW, Kang SH, Kim MY, Lee JI, Baik SK. Mesenchymal stem cells to treat liver diseases. Ann Transl Med. 2020;8(8):563. Available from: https://doi.org/10.21037%2Fatm.2020.02.163
- Mäkelä T, Takalo R, Arvola O, Haapanen H, Yannopoulos F, Blanco R, et al. Safety and biodistribution study of bone marrow-derived mesenchymal stromal cells and mononuclear cells and the impact of the administration route in an intact porcine model. Cytotherapy. 2015;17(4):392-402. Available from: https://doi.org/10.1016/j.jcyt.2014.12.004
- Kim I, Bang SI, Lee SK, Park SY, Kim M, Ha H. Clinical implication of allogenic implantation of adipogenic differentiated adipose-derived stem cells. Stem Cells Transl Med. 2014;3(11):1312-1321. Available from: https://doi.org/10.5966%2Fsctm.2014-0109