More Information
Submitted: October 04, 2024 | Approved: October 10, 2024 | Published: October 11, 2024
How to cite this article: Luisetto M, Mashori GR, Cabianca L, Latyshev OYU. Budesonide – Ora Galenic Formulations for Crohn Disease. Ann Clin Gastroenterol Hepatol. 2024; 8(1): 028-033. Available from: https://dx.doi.org/10.29328/journal.acgh.1001048.
DOI: 10.29328/journal.acgh.1001048
Copyright License: © 2024 Luisetto M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Budesonide; Crohn's disease; Pediatric; Delay release; Capsules acid resistance filled with HPMC; Metolose; Methocel; Oral suspension; Ready for use vehicle; 3D printing
Budesonide – Oral Galenic Formulations for Crohn Disease
Luisetto M1*, Mashori GR2, Cabianca L3 and Latyshev OYU4
1Independent Researcher Applied Pharmacologist, Hospital Pharmacist Manager, Marijnskaya, PC Area 29121, Italy
2Professor, Department of Medical & Health Sciences for Woman, Peoples University of Medical and Health Sciences for Women, Pakistan
3Medical Laboratory, Turin, City of health, Italy
4IMA President, Italy
*Address for Correspondence: Luisetto M, Independent Researcher Applied Pharmacologist, Hospital Pharmacist Manager, Marijnskaya, PC Area 29121, Italy, Email: [email protected]
The aim of this work is to verify the pharmaceutical form in the galenic field of oral Budesonide compounded used in Crohn’s disease: capsules delay release or oral suspension. In particular ways the kinds of excipients or bases-vehicle used in the galenic pharmacy practice. The therapeutic need for Crohn’s disease requires a release of the API in delayed-release DR. The Budesonide molecule shows low systemic impacts due to its hepatic metabolism vs. a topical effect useful in this pathology. In this work, the oral pharmaceutical forms are analyzed: modified-release capsules and oral suspension with specific advantages for each one. Some formulations provided by various pharmacies are reported in this work as well as new technology like the 3D-PRINTING systems for colonic targeting tablets.
Crohn‘s disease can affect both children and adults: as reported by Kelsen and Baldassano in 2008, “Inflammatory Bowel Disease (IBD) is a group of diseases that include Crohn's disease and ulcerative colitis. Presenting symptoms and therapeutic options are similar in adult and pediatric patients. But there are significant differences in the 2 populations that require separate approaches to treatment and management of the disease in children. IBD is now being recognized with increased frequency in both adults and in children of all ages” [1].
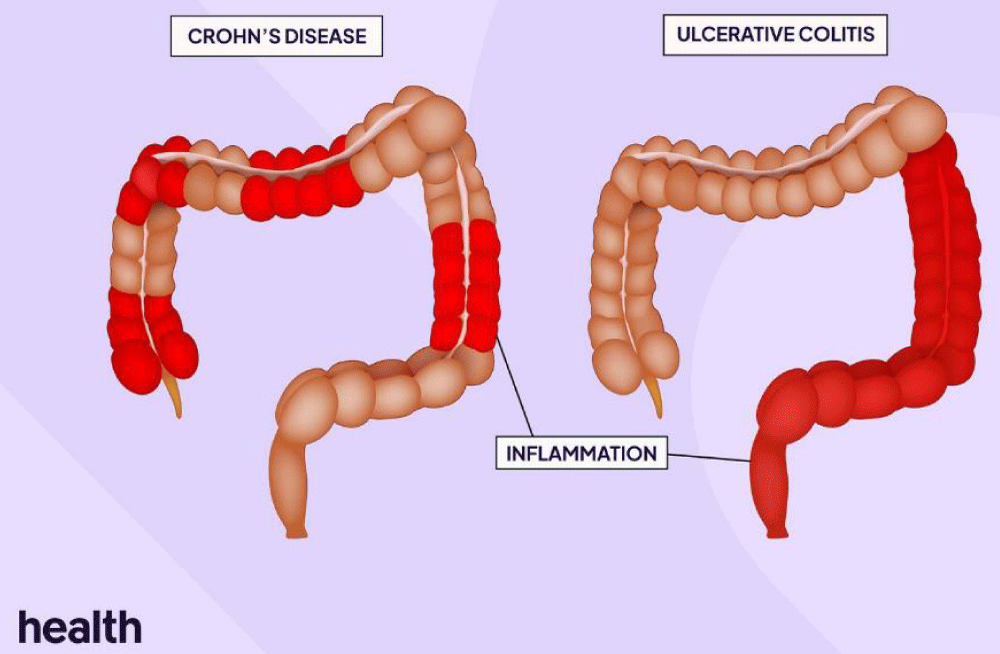
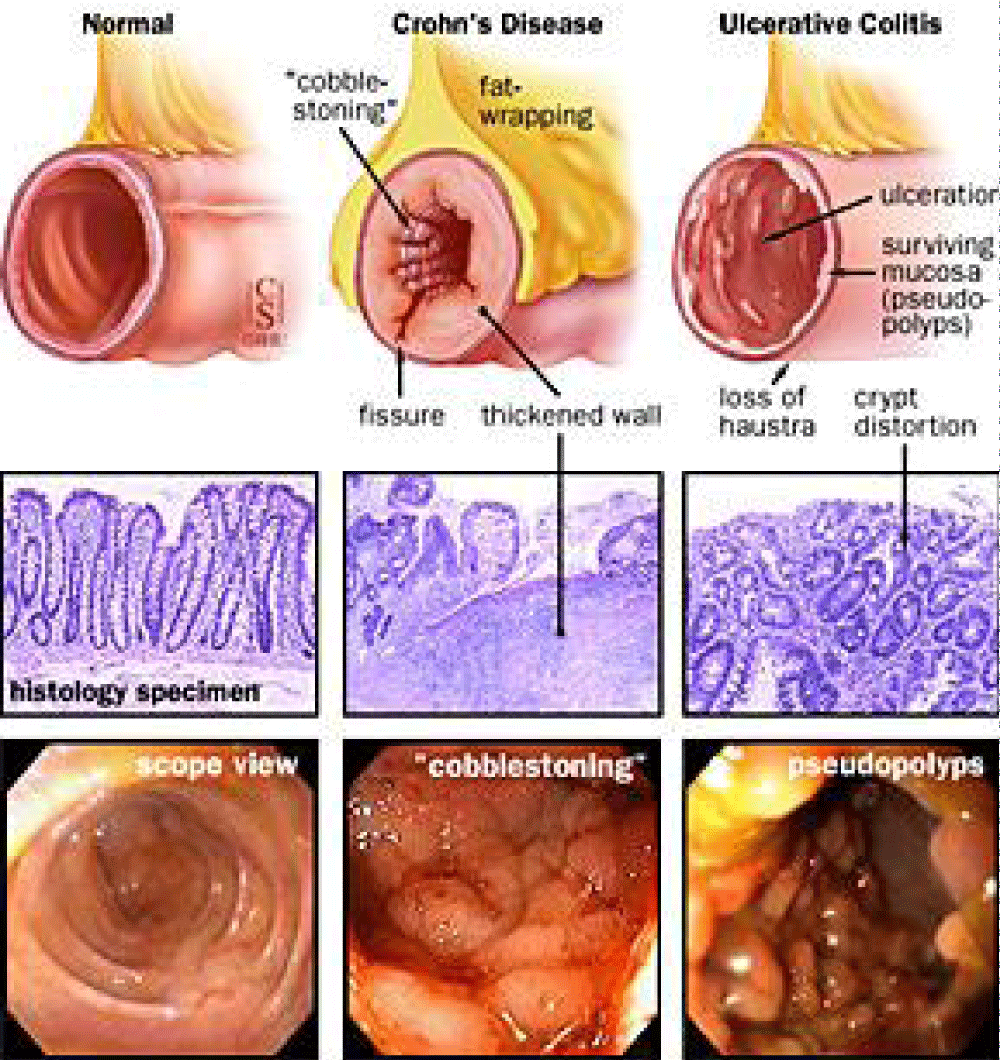
The related pathology characteristics as reported by Feuerstein and Cheifetz is that “Crohn's disease is a chronic idiopathic inflammatory bowel disease IBD condition characterized by skip lesions and transmural inflammation that can affect the entire gastrointestinal tract from the mouth to the anus.” [2] (Figures 1,2).
Figure 1: Crohn’s disease and ulcerative colitis [3].
Figure 2: Crohn’s disease pathology [4].
The related epidemiology and incidence as addressed by von Allmen in 2018 is that “the incidence of Crohn's disease CD in the pediatric population is increasing. While pediatric patients with Crohn's disease exhibit many of the characteristics of older patients, there are important differences in the clinical presentation and course of disease that can impact the clinical decisions made during treatment.
The majority of children are diagnosed in the early teen years, but subgroups of very early onset and infantile Crohn's present much earlier and have a unique clinical course” [5].
Between the various therapeutic options before the introduction of biological drugs, a review by Kumar, et al. stated that “Truelove and Witts first demonstrated the efficacy of corticosteroid treatment in acute severe UC in 1955. Corticosteroids, however, have numerous unwanted side effects, such as metabolic (steroid-induced diabetes, cushingoid appearance, and hepatic steatosis), central nervous system (psychosis, insomnia, and emotional disturbances), gastrointestinal GI (dyspepsia and peptic ulcer), musculoskeletal (osteonecrosis of the jaw and hip, osteoporosis, and growth failure), skin (easy bruising, skin thinning, weight gain, acne, hirsutism, striae, and purpura), and ocular effects (glaucoma and cataracts). Long-term use can also increase the risk of infection, lead to impaired wound healing, and can result in steroid dependence. In 1994, a newer glucocorticoid formulation, budesonide, was shown to have equal efficacy to prednisolone, 16 with a 15 times greater affinity for glucocorticoid receptors, such that 5 mg of budesonide is equivalent to 12 mg of prednisolone. Budesonide has an added advantage of a high first pass liver metabolism and rapid elimination, resulting in minimal systemic absorption and thereby reducing the risk of steroid-induced side effects.” [6] (Figure 3).
Figure 3: Drugs used in CD [6].
Budesonide for Crohn's Disease
Gordon R. Greenberg, et al. in 1994 stated “Budesonide is a corticosteroid with high topical antiinflammatory activity but low systemic activity because of extensive hepatic metabolism” [7].
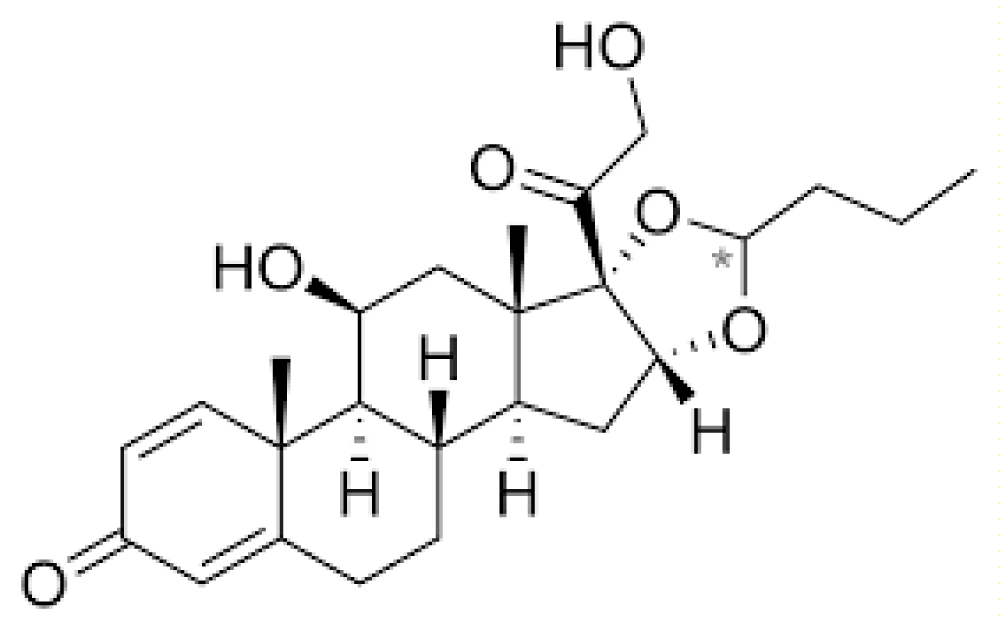
Regarding the formulation in use, it is possible to see on the Bayview Pharmacy website that “The Budesonide 2 mg/10 ml Oral Suspension OS is available in a liquid dosage form. This form allows for the ingredients to be dispersed uniformly throughout a liquid medium, providing a homogeneous mixture for administration. This makes it easy to take and measure the correct dose. It is crucial to take Budesonide exactly as prescribed by your doctor.” [8] The chemical structure is shown in Figure 4.
Figure 4: Budesonide - chemical structure formula.
Generally, the Budesonide dosage forms can be an oral capsule, extended-release (6 mg; 9 mg), oral delayed-release capsule (3 mg; 4 mg), oral suspension (2 mg/10 mL), oral tablet, extended-release (9 mg) and so on.
The dosing as recommended by Mayo Clinic for Budesonide in Crohn’s disease is as follows:
“For oral dosage form (delayed-release capsules):
For mild to moderate active Crohn's disease:
Adults-9 milligrams (mg) once a day in the morning for up to 8 weeks. Your doctor may adjust your dose as needed.
Children 8 to 17 years of age and weighing more than 25 kilograms (kg)—At first, 9 mg once a day in the morning for up to 8 weeks, followed by 6 mg once a day in the morning for 2 weeks.
Children younger than 8 years of age or weighing 25 kg or less—Use and dose must be determined by your doctor.
For prevention of symptoms of Crohn's disease from coming back:
Adults-6 milligrams (mg) once a day in the morning for up to 3 months. Your doctor may adjust your dose as needed.
Children-Use and dose must be determined by your doctor”. [9]
Observing the Budesonide Te Arai 3 mg controlled-release capsules technical sheet, the List of excipients [10] mentions the following:
Capsule content
Sugar pellets (Maize starch & Sucrose)
Ethyl cellulose Dispersion Type B
Polysorbate 80
Methacrylic acid polymer type C
Triethyl citrate
Talc
Capsule shell
Black iron oxide E172
Red Iron Oxide E172
Titanium dioxide E171
Gelatin
In the ‘Therapeutic benefits of budesonide in gastroenterology’ by O'Donnell and O'Morain, “Budesonide is a synthetic steroid of the glucocorticoid family with a high topical anti-inflammatory activity. Enteric-coated EC formulations resist gastric-acid degradation, delivering active drug to the small intestine and proximal colon” [12].
In ‘Colon-targeted delivery systems of budesonide as second-line therapy in inflammatory bowel disease’, Hennig and Hennig mentioned that “To deliver BUD to the colon, the drug formulation should be formulated so that it prevents the release of the drug in the upper GIT and starts releasing the drug content as soon as it reaches the colon. Various approaches, including the modifying of pharmaceutical formulations using drug delivery systems DDS dependent on microbial degradation, time-dependent and pH-dependent, have been investigated separately or in combination with each other”. [11] (Figure 5).
Figure 5: Budesonide cps, GI pH variation, and delivery [11].
With an observational method, some relevant literature (from 1 to 10) is reported related to the topic of this work. Various figures (from 1 to 10) help better understand the concepts. An experimental project hypothesis is reported and finally, a global conclusion is submitted after analyzing all.
From the literature and professional websites, the following is presented:
On the Bayview Pharmacy website for Budesonide 10 mg Slow Release Acid Resistant Capsules, “Budesonide 10 mg Slow Release Acid Resistant Capsules, formulated with Methocel E4M, are designed to gradually release the active ingredient over an extended period. This controlled-release mechanism offers sustained therapeutic effects, reduces dosing frequency, and improves patient compliance. These capsules are resistant to stomach acid and are used to treat conditions such as Asthma, Crohn's Disease, Ulcerative Colitis, Allergic Rhinitis, and Eosinophilic Esophagitis.
The acid-resistant AR feature of the capsules protects the medication from being degraded in the stomach, thereby enhancing absorption and improving the overall efficacy of the drug. This ensures that the medication is delivered to the site of inflammation in the body, providing relief from symptoms and reducing inflammation.
What is the purpose of the Methocel E4M in the formulation?
Methocel E4M is a type of controlled-release polymer. It is used in the formulation to ensure that the medication is released gradually over an extended period of time. This offers sustained therapeutic effects and reduces the frequency of dosing.” [13].
From the Textbook of Pharmaceutical Excipients (Fifth edition), “ synonyms Benecel MHPC; E464; hydroxypropyl methylcellulose; HPMC; Methocel; methylcellulose propylene glycol ether; methyl hydroxypropylcellulose; Metolose; TylopurIn oral products.
Hypromellose is primarily used as a tablet binder,(1) in film-coating,(2–7) and as a matrix for use in extended-release tablet formulations. (8–12) Concentrations between 2% and 5% w/w may be used as a binder in either wet- or dry-granulation processes. High-viscosity grades may be used to retard the release of drugs from a matrix at levels of 10% – 80% w/w in tablets and capsules” [14].
About the Budesonide CPS from a compounding pharmacy service in the USA, they provided that, “We put budesonide in an acid resistant capsule, then use a 40% blend of Hydroxypropyl Methylcellulose as a filler to help delay the release of budesonide.”
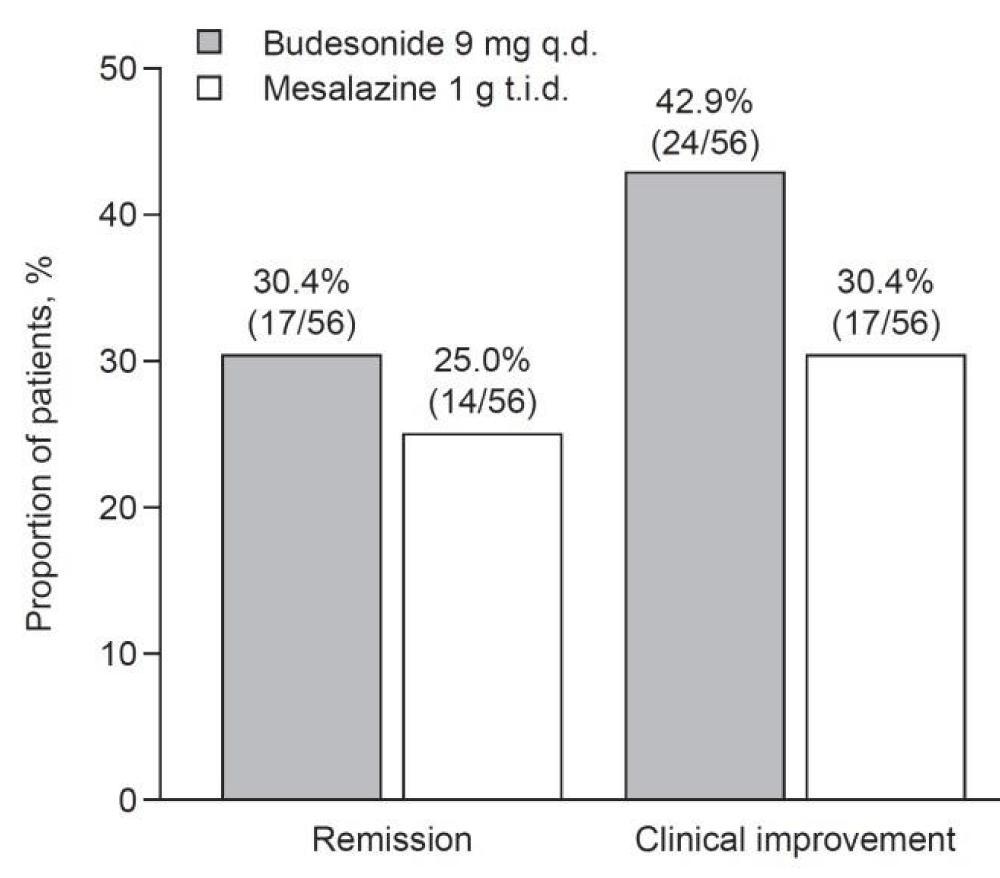
According to Tadashi Yokoyama, et al. “Primary Assessments. The proportion of patients who achieved remission at week 8 was numerically higher in the budesonide group than in the mesalazine group (30.4 vs. 25.0%; p = 0.526;...” as shown in Figure 6 [15].
Figure 6: Rates of remission (Crohn's Disease Activity Index [CDAI] score ≤150) and clinical improvement (CDAI score ≤150 or CDAI score decrease from baseline ≥100) at week 8 of the treatment phase. q.d., once daily; t.i.d., three times daily [15].
Ashish Chopra, et al. state that, “Delayed-release budesonide (Entocort EC) is enteric coated and designed to deliver budesonide to the terminal ileum and proximal colon, where Crohn's disease is most common.” [16].
According to the study by Yi Hsuan Ou, et al. they state, “In this study work, we have demonstrated the ability to engineer 3D printed pill-in-pill (CORR3CT) tablets to target specific sites along the gastrointestinal tract, in particular the colon. The 3D printed tablets are also comparable to commercially available budesonide oral.” [17].
Rita Cortesi, et al. mentioned that “Eudragit®RS microparticles showed a better protection of the drug from gastric acidity than those of Eudragit®RS/Eudragit®RL allowing us to propose Eudragit®RS micro-particles as a hypothetical system of colon specific controlled delivery.” [18].
It was stated in a study by Iborra M, et al. that “Budesonide is available in three oral dose forms: a controlled ileal release form, a pH-dependent release formulation, and a MMX formulation.
Both controlled ileal and pH-dependent release use enteric coated (Eudragit®, Evonik Industries) pellets and have been approved for treating CD located in the terminal ileum and/or ascending colon. The controlled ileal release form (Entocort®, AstraZeneca, ; Entocir®, Sofar SpA) contains L 100-55 Eudragit® granules, which are resistant to gastric acid degradation and dissolve at pH values above 5.5. A pH-dependent release formulation (Budenofalk®, Dr. Falk Pharma) is an enteric coated locally acting glucocorticoid preparation whose pH- and time-dependent coating enables its release into the ileum and ascending colon. This oral formulation consists of a capsule containing L, S, LS, and RS Eudragit® granules that dissolve at pH values above.
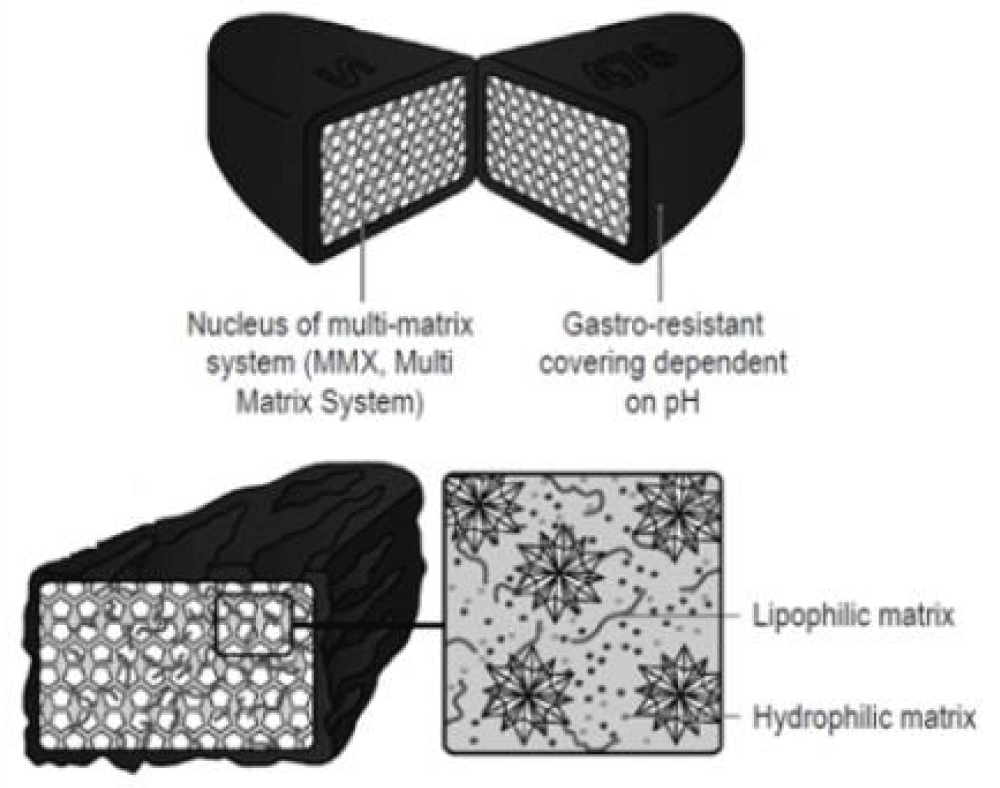
A new controlled release system, Budesonide MMX® (Cosmo Pharmaceuticals SpA, Lainate, Italy), has recently been developed and researched. MMX technology comprises hydrophilic and lipophilic excipients, both of which are enclosed within a gastroresistant and pH-dependent coating” [19].
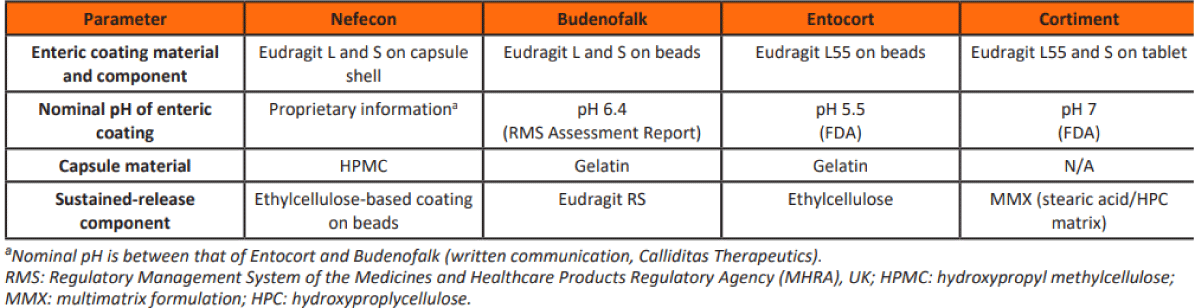
According to Jennifer Dressman, et al. “Prolonged (extended) release of budesonide is ensured by embedding the drug in a multimatrix (MMX) formulation” [20] (Figures 7- 9).
Figure 7: Pharmaceutical characteristics of DR Budesonide Oral formulations [20].
Figure 8: Comparative Dissolution of Budesonide from Four Commercially Available Products for Oral Administration [20].
Figure 9: Matrix MMX system [21].
Fouad S. Moghrabi, et al. stated that “To date, several enteric, ready-to-fill capsules are commercially available, which claim to prevent gastric drug release. These include: Bio-VXR® (BioCaps) with an undisclosed formulation of vegetable capsules, DRcapTM (Lonza Capsules and Health Ingredients) nutraceutical capsules composed of HPMC and gellan, designed to swell and delay disintegration, enTrinsicTM drug delivery capsules (Lonza) composed of Cellulose Acetate Phthalate (CAP) and Vcaps® Enteric capsules (Lonza) composed of HPMC, HPMC-AS polymers and gellan gum as the gelling agent. In 2021, EUDRACAPTM (Evonik, Darmstadt) HPMC capsules coated with methacrylic acid copolymers that can easily be opened and closed were launched.” [22].
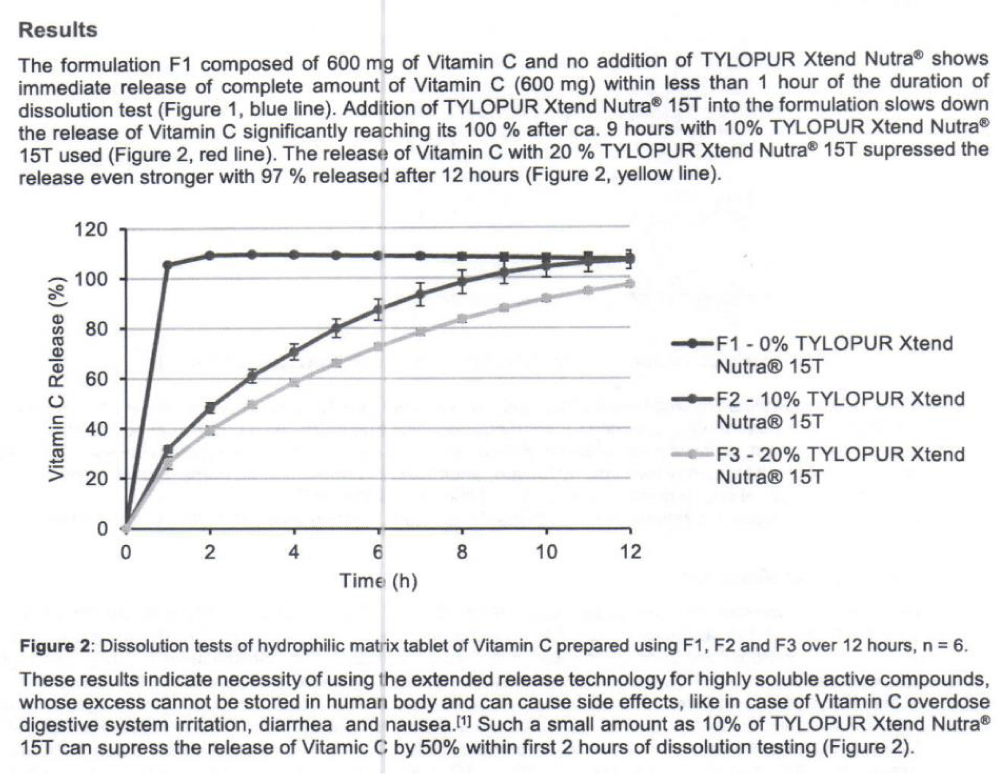
To be observed in nutraceutical setting also, in the technical information of Extended Release of Vitamin C Matrix Tablets with TYLOPUR Xtend Nutra®, “WLOPUR Xtend Nutra@ is an excellent choice as a highly compressible, hydrophilic matrix agent for nutraceutical and nutritional tablet applications. Straight forward and easy direct compression formulation of extended rèlease hydrophilic matrix tablets of natural active compounds (Vitamin C used here as an example) using TYLOPUR Xtend Nutra@ is cost effective. The results show that TYLOPUR Xtend Nutra@ regulates the release of Vitamin C in a controlled manner, slowing it significantly depending on the amount used”. [23] (Figure 10).
Figure 10: Dissolution test of hydrophilic matrix tablet of vit. C prepared using various formulations [23].
Experimental project hypothesis
To verify the efficiency of the use of AR CPS filled with API mixed in Methocel E4M (40%) is needed.
To test the level of the API after 1-2 h in an acidic environment with a pH similar to gastric fluids and after a buffered medium-like intestinal pH.
If the system tested really protects the gastro sensible API the matrix methods can be used for this scope.
Budesonide is currently used in the therapy of Crohn’s disease or other inflammatory conditions. This kind of condition shows low systemic toxicity and good topical efficacy: this is due to extensive liver metabolism. Various strategies are used by the producers to provide a delayed release to protect from gastric fluids degradation: kind of capsules, enteric coating of the capsules, matrix systems (ex hydroxipropilcellulose based). In current therapy, there are various formulations available: from capsules slow- delay release – acid resistance or also in oral suspension. Interestingly, the cps AR filled with the API in Methocel E4M (about 40%) a controlled-release polymer is used by some pharmacies. The limitation of this work is related to the need to test in the laboratory the goodness of the method that uses AR CPS filled with Budesonide in a mix of excipients (about 40% methocel) related to the efficacy API release into the intestine. It is needed to verify this with future studies even if some compounding centres use this method.
It is fundamental for the therapy of Crohn’s disease with BUDESONIDE to use a delayed-release oral pharmaceutical form in order to protect from the gastric acid pH. (Generally, the registered drugs are gastroresistance pellets inside normal capsules). Various formulations are used, of interest is the use of AR CPS filled with Methocel 40% to delay the release of the API in the intestinal setting and the oral suspension (as a versatile pharmaceutical form).
- Kelsen J, Baldassano R N. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008;14(suppl_2):S9-11. Available from: https://doi.org/10.1002/ibd.20560
- Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017;92(7):1088-1103. Available from: https://doi.org/10.1016/j.mayocp.2017.04.010
- Brandi Jones M-E, Rekhi S. What’s the difference between Crohn’s disease and ulcerative colitis? Health. 2024 Feb 7. Available from: https://www.health.com/crohns-disease-vs-ulcerative-colitis-8405457
- Crohn’s Disease. Johns Hopkins Medicine. 2022 Apr 19. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/crohns-disease
- von Allmen D. Pediatric Crohn's disease. Pediatr Congenit Colorectal Dis Adult Patient. 2018;31(2):80-88. Available from: https://doi.org/10.1055/s-0037-1609022
- Kumar A, Cole A, Segal J, Smith P, Limdi JK. A review of the therapeutic management of Crohn’s disease. Ther Adv Gastroenterol. 2022;15:17562848221078456. Available from: https://doi.org/10.1177/17562848221078456
- Greenberg G R, Feagan B G, Martin F, Sutherland L R, Thomson A, Williams C N, et al. Oral budesonide for active Crohn's disease. N Engl J Med. 1994;331(13):836-841. Available from: https://doi.org/10.1056/nejm199409293311303
- Budesonide 2 mg/10 ml Oral Suspension | Formulas | Bayview Pharmacy. Available from: https://www.bayviewrx.com/formulas/Budesonide-2-mg-10-ml-Oral-Suspension-Asthma-Allergic-Rhinitis-Crohn-s-Disease-Ulcerative-Colitis-Eosinophilic-Esophagitis
- Budesonide (oral route). Mayo Clinic. 2024 Oct 1. Available from: https://www.mayoclinic.org/drugs-supplements/budesonide-oral-route/description/drg-20073233
- Budesonide Te Arai. Budesonide Te Arai Data Sheet v 3.0 July 2023. Available from: https://www.medsafe.govt.nz/profs/datasheet/b/BudesonideTeAraicap.pdf
- Hennig M, Hennig M. Colon-targeted delivery systems of budesonide as second-line therapy in inflammatory bowel disease. Pharma Excipients. 2024 Feb 25. Available from: https://www.pharmaexcipients.com/news/budesonide-second-line/
- O'Donnell S, O'Morain CA. Therapeutic benefits of budesonide in gastroenterology. Ther Adv Chronic Dis. 2010;1(4):177-186. Available from: https://doi.org/10.1177/2040622310379293
- Budesonide 10 mg Slow Release Acid Resistant Capsules | Formulas | Bayview Pharmacy. Available from: https://www.bayviewrx.com/formulas/Budesonide-10-mg-Slow-Release-Acid-Resistant-Capsules-Asthma-Crohn-s-Disease-Ulcerative-Colitis-Allergic-Rhinitis-Eosinophilic-Esophagitis
- Rowe RC, Sheskey PJ, Owen SC. Handbook of Pharmaceutical Excipients. 5th ed. Pharmaceutical Press; 2006;918. https://books.google.co.in/books/about/Handbook_of_Pharmaceutical_Excipients.html?id=BGJqAAAAMAAJ
- Yokoyama T, Ohta A, Motoya S, Takazoe M, Yajima T, Date M, et al. Efficacy and safety of oral budesonide in patients with active Crohn's disease in Japan: a multicenter, double-blind, randomized, parallel-group phase 3 study. Inflamm Intest Dis. 2018;2(3):154-162. Available from: https://doi.org/10.1159/000484047
- Chopra A, Pardi DS, Loftus EV, Tremaine WJ, Egan LJ, Faubion WA, et al. Budesonide in the treatment of inflammatory bowel disease: the first year of experience in clinical practice. Inflamm Bowel Dis. 2006;12(1):29-32. Available from: https://doi.org/10.1097/01.mib.0000192323.82426.83
- Ou YH, Goh WJ, Lim SH. Form & formulation approaches for COntRollable Release in 3D printed Colonic Targeting (CORR3CT) budesonide tablet. Int J Pharm. 2023;635:122680. Available from: https://doi.org/10.1016/j.ijpharm.2023.122680
- Cortesi R, Ravani L, Menegatti E, Esposito E, Ronconi F. Eudragit® microparticles for the release of budesonide: a comparative study. Indian J Pharm Sci. 2012;74(5):415-421. Available from: https://doi.org/10.4103/0250-474x.108416
- Iborra M, Álvarez-Sotomayor D, Nos P. Long-term safety and efficacy of budesonide in the treatment of ulcerative colitis. Clin Exp Gastroenterol. 2014;39-46. Available from: https://doi.org/10.2147/ceg.s34715
- Dressman J. Comparative dissolution of Budesonide from four commercially available products for oral administration: implications for interchangeability. Dissol Technol. 2023;30:224-229. Available from: https://dissolutiontech.com/issues/202311/DT202311_A02.pdf
- Management. MMXTM technology and its applications in gastrointestinal diseases - Tasnim Pharmaceutical Company. Tasnim Pharmaceutical Company. 2023 Jul 19. Available from: https://tasnimpharma.com/mmx-technology/
- Moghrabi FS, Fadda HM. Drug physicochemical properties and capsule fill determine extent of premature gastric release from enteric capsules. Pharmaceutics. 2022;14(11):2505. Available from: https://doi.org/10.3390/pharmaceutics14112505
- Shin-Etsu technical information - SE Tylose. Extended Release of Vitamin C Matrix Tablets with TYLOPUR Xtend Nutra®. Available from: https://www.setylose.com/en/knowledge-base/healthcare/technical-information#tylopur-xtend-nutra-10231